Editor’s Note: Unlike aluminum’s sustainable advantage in recycling, primary aluminum production (which uses carbon anodes in the Hall-Héroult process), continues to be energy intensive and a greenhouse gas (GHG) producer. Ignoring the effect of CO2 generation in the coal/hydrocarbon power equation, the use of carbonaceous anodes in the smelting process generates GHGs as carbon is consumed to produce CO2 in the anode reaction, amounting to ~1.5 t CO2/t Al, i.e., 50% more CO2 is produced than Al in the more efficient smelters. Perfluorocarbons (PFCs) are also produced in the aluminum reduction process during anode effects, which are unwanted process related conditions resulting from insufficient amounts of aluminum oxide dissolved in the electrolytic fluoride salt bath within the potlines causing voltage spikes and the emission of PFC-containing gases. Although primary aluminum producers strive to minimize anode effects in their smelters, effects of emitted PFCs have greenhouse warming effects that are thousands of times as strong as those of CO2 and atmospheric lifetimes of some 10,000 years (“Aluminum Industry,” EPA).
For more than a century it has been realized that inert or non-consumable anodes made of materials that have the requisite high temperature conductivity and resistance to attack by the molten fluoride salts in today’s aluminum smelters evolve O2 (1 t O2/t Al) rather than CO2 and PFCs during electrolysis. In fact, among the five U.S. patents granted to Charles M. Hall on April 2, 1889 for his discovery of extracting aluminum from its oxide, one in particular—U.S. 400,664 (Process for Reducing Aluminium from Its Fluoride Salts by Electrolysis)—considers the development of inert anodes using copper. These copper-based anodes did not work, as copper dissolved in the electrolyte and aluminum metal that was reduced at the cathode.
For decades since Hall’s experiments with inert anodes, aluminum electrolysis using inert anodes has been and continues to be a prime research target by all the major aluminum companies worldwide. Since Hall’s invention, hundreds of international patents have been granted on inert anode technology to some of the largest aluminum companies in the world, as well as to independent inventors. These numerous and diverse patents are indicative of the R&D efforts expended at international aluminum companies on new inert anode materials, inert anode manufacture, special electrolyte compositions used with inert anodes, cell designs, electrical connections to the inert anodes, thermal shock mitigation, and process controls. The recent inert anode patents presented here mark the evolution of current ideas aimed at solving some of the problems that have kept inert anode technology seemingly forever trapped in the development phase.
Most recently, the inert anode technology developed by the joint venture Elysis, led by Rio Tinto and Alcoa and supported by Apple, could have not only an environmental benefit, but also energy, cost, and productivity benefits as well. Elysis partner Alcoa had invested heavily in inert anode technology in the 1990s and later boasted of a full-scale test sometime in early 2000. The Alcoa project was a secretive project, but the experience gained and many patents granted to Alcoa on inert anode technology will supplement Rio Tinto’s technology to make for a formidable combination. No doubt, this will drive inert anode technology into an advanced pilot stage and more.
No less important than inert anodes in advancing the state of aluminum electrolysis is wetted cathode technology, which also promises energy reducing and environmental and production enhancing benefits. By creating a cathode surface that is inert and wettable to the molten aluminum pad, which acts as the cathode for the electrolysis reaction, the anode-cathode distance can be reduced by half or more, thereby reducing the voltage drop with substantial energy savings. In fact, wettable cathodic materials, e.g., TiB2, can achieve energy reductions in aluminum electrolysis greater than that of inert anodes when used alone; inert anodes combined with wettable cathodes are the most energy efficient electrodes to use in aluminum electrolysis (www.mdpi.com/journal/sustainability).
The international patent survey presented here on inert anode technology and wettable cathodes showcases the recent inventions developed by Elysis and other international aluminum companies. Also included is the Beck electrolysis cell patent abstract (US8480876), exploiting vertical inert anodes and cathodes at a lower electrolyte temperature, which is the basis of R&D work on inert anodes by Arctus Metals Ltd. in Iceland (indicating a role for the individual inventor in this quest). The focus on green and energy efficient anode and cathode technologies represents a sea change for the primary aluminum industry going forward. Capital providers are adhering to the UN’s Sustainable Development Goals (SDGs), which will require an environmental alignment of the industry beyond recycling for survival in the coming decade.
The quality investors and size of their investment in Elysis represent a recognition of the changing regulatory climate for the primary aluminum industry and a needed and positive adaptation to that climate. As a prediction: a production level operation for truly “green primary aluminum” utilizing inert anodes, ideally with wettable cathodes, will eventually be built, and it may well be in Canada.
– Joseph C. Benedyk, Editor
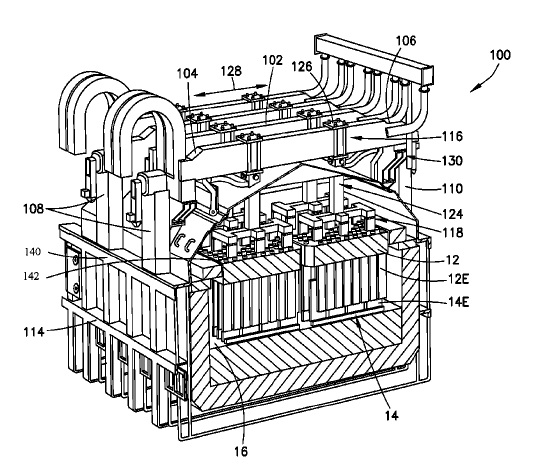
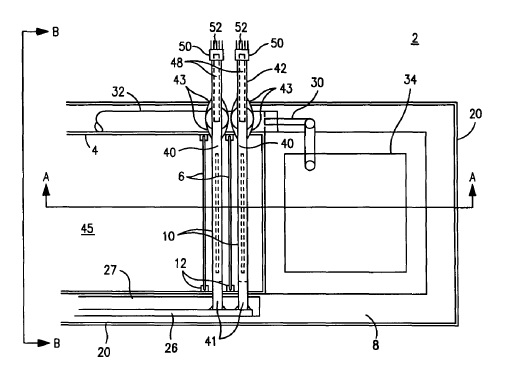
US10415147 — ELECTRODE CONFIGURATIONS FOR ELECTROLYTIC CELLS AND RELATED METHODS — Elysis Limited Partnership (Canada) — In one embodiment, an electrolytic cell for the production of aluminum from alumina includes: at least one anode module with each module configured with a plurality of vertically oriented inert anodes; at least one cathode module, opposing the anode module, wherein the at least one cathode module comprises a plurality of cathodes, wherein the plurality of anodes are suspended above the cathode module and extending downwards towards the cathode module, wherein the plurality of cathodes are positioned extending upwards towards the anode module, wherein each of the plurality of anodes and each of the plurality of cathodes are alternatingly positioned, wherein the plurality of anodes is selectively positionable in a horizontal direction relative to adjacent cathodes, wherein the anode module is selectively positionable in a vertical direction relative to the cathode module, and wherein a portion of each of the anode electrodes overlap a portion of adjacent cathodes.
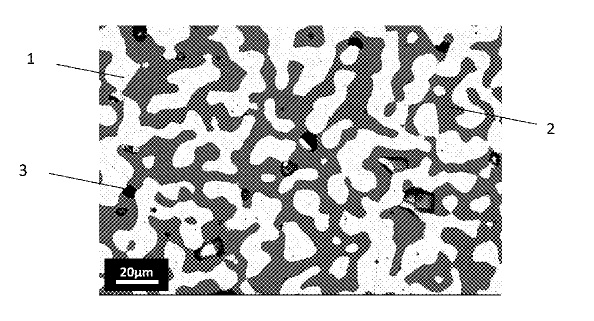
US10415122 — CERMET ELECTRODE MATERIAL — Elysis Limited Partnership (Canada) — A cermet material includes as mass percentages, at least: 50% to 90% of a metallic phase containing an alloy of copper (Cu) and nickel (Ni), and 10% to 50% of an oxide phase containing at least iron, nickel and oxygen with the following proportion by mass of Ni: 0.2%<Ni<17%. An electrode, preferably an anode, may include this cermet material.
US9982355 — SYSTEMS AND METHODS FOR PREVENTING THERMITE REACTIONS IN ELECTROLYTIC CELLS — Alcoa USA Corp. (USA) — In some electrolytic cells, the use of substantially “non-consumable” or “inert” anodes offer a cost effective and more environmentally sound alternative to carbon anodes. However, when the inert anode includes metal oxides, there is a possibility of a thermite reaction between the metal oxides and the aluminum metal in the electrolysis cell, leading to possible cell failure or cell eruption. A method of monitoring an electrolytic cell including detecting information indicative of a thermite reaction, comparing the information indicative of a thermite reaction to a threshold, generating a thermite response signal according to the comparison, and reacting to the thermite response signal by adjusting the operation of the electrolytic cell.
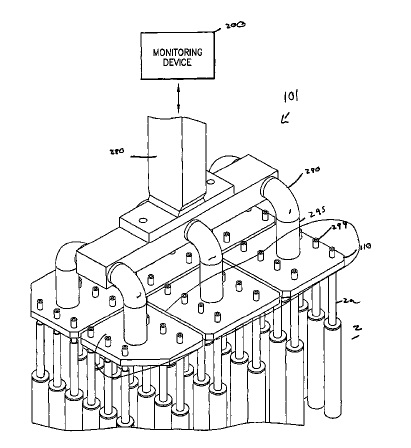
US9945041 — ANODE APPARATUS — Alcoa USA Corp. (USA) — The present disclosure is related to an inert anode which is electrically connected to the electrolytic cell including a pin where the pin extends into the anode body to a certain location (e.g. depth into a hole in the anode body), such that a conductor rod is connected to the inert anode in order to supply current from a current supply to the inert anode, where the inert anode directs current into the electrolytic bath to produce nonferrous metal (where current exits the cell via a cathode), where the pin extends into the anode body to a certain portion of the total length of the anode body, and is positioned inside the anode such that during operation of the anode (i.e. in an electrolysis cell to produce non-ferrous metal), the pin is above the bath-vapor interface.
US9738983 — METHOD FOR FABRICATING A DENSE, DIMENSIONALLY STABLE, WETTABLE CATHODE SUBSTRATE IN SITU — KCL Enterprises, LLC (USA) — Compositions suitable for use in an electrolytic cell for producing aluminum are provided. The compositions can contain a powder blend of boron oxide, a titanium dioxide, aluminum, and titanium diboride. The powder blend can be compacted into tiles and arranged as a cathode surface. The boron oxide and the titanium dioxide in the tiles can be made to react under low temperature molten aluminum to produce titanium diboride in situ. The reaction yields a dense dimensionally stable wettable cathode substrate that can reduce the power consumption in the aluminum electrowinning process.
US9598783 — ALUMINUM SMELTER COMPRISING ELECTRICAL CONDUCTORS MADE FROM A SUPERCONDUCTING MATERIAL — Rio Tinto Alcan International Limited (Canada) — An aluminum smelter comprising: (i) a series of electrolytic cells, designed for the production of aluminum, forming one or more rows, (ii) a supply station designed to supply the series of electrolytic cells with an electrolysis current, the said electricity supply station comprising two poles, (iii) a main electrical circuit through which the electrolysis current flows, having two extremities each connected to one of the poles of the supply station, (iv) at least one secondary electrical circuit comprising an electrical conductor made of superconducting material through which a current flows, running along the row or rows of electrolytic cells, characterized in that the electrical conductor made of superconducting material in the secondary electrical circuit runs along the row or rows of electrolytic cells at least twice in such a way as to make several turns in series. In particular, the invention may extend to aluminum smelter using electrolysis with inert anodes.
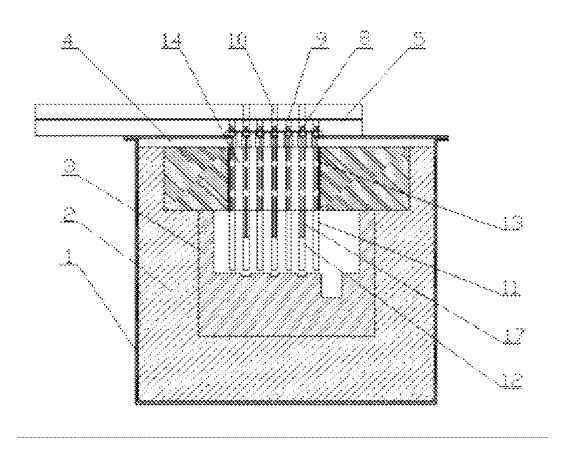
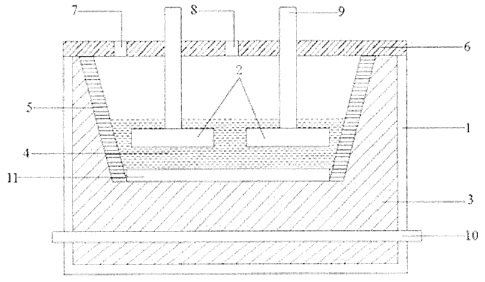
US9551078 — ELECTROLYTIC CELL FOR PRODUCING PRIMARY ALUMINUM BY USING INERT ANODE — Aluminum Corporation of China Limited (China) — An electrolytic cell for producing primary aluminum by using inert anodes is disclosed, in which an electrolyte system KF–NaF–AlF.sub.3 is used and the operating temperature of the cell is 700-850.degree. C. The electrolytic cell comprises a cell shell, heat insulating refractory lining, a melting pot, a heat insulating cover, inert electrodes, electrode stems, anode bus-bars, cathode bus-bars, anode branching bus-bars, heat insulating plates, partitions between anodes and cathodes and a feeding device. The quality of the aluminum product obtained by using the electrolytic cell is not less than 99.7%. The cell is free from emission of carbon dioxide and perfluorinated compounds (PFCs), and hardly has consumption of electrodes, so the distances between anodes and cathodes can be kept stable. The cell is sealed and the volatilization of dust and fluorides can be prevented, and it is useful to recover oxygen gas.
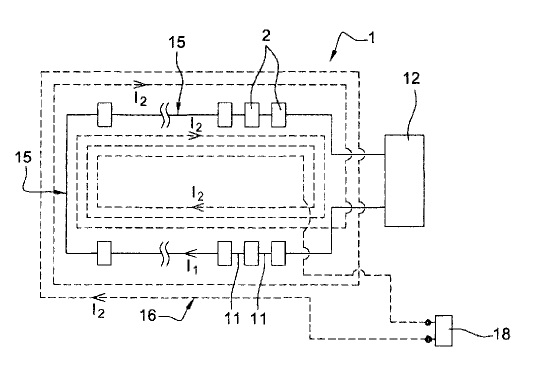
US9528193 — DIRECT-CURRENT SHUNT PREHEATING START METHOD FOR AN INERT ELECTRODE ALUMINUM ELECTROLYSIS CELL — Aluminum Corporation of China Limited (China) — The invention discloses a direct-current shunt preheating start method for an inert electrode aluminum electrolysis cell, comprising: (1) forming multiple groups of direct-current shunt elements by using conductors with preset resistance values and geometric sizes; (2) laying in a hearth of the electrolysis cell electrical heating element groups of the same number as/a different number from electrode groups; (3) drying the hearth, smelting electrolyte and establishing a thermal balance and a hearth inner profile by using the electrical heating element groups according to a set heating curve or set steps; (4) changing the number of groups/a series or parallel connection state of the direct-current shunt elements; and (5) gradually replacing inert electrodes and gradually adjusting the number of the groups of/the series or parallel connection state of the shunt elements. By means of the present invention, the inert electrode aluminum electrolysis cell can be well preheated and the thermal balance can be established; in the inert electrode replacement process, stability of the cell voltage can further be ensured, so that the current passing through the inert electrodes in the cell is uniform; and series current is not affected by start of a single electrolysis cell, so that non-disturbance start is implemented.
US9217204 — CONTROL OF TEMPERATURE AND OPERATION OF INERT ELECTRODES DURING PRODUCTION OF ALUMINUM METAL — Norsk Hydro ASA (Norway) — The present invention relates to methods for operating and controlling the temperature of inert electrodes during production of molten aluminum by electrolysis of an aluminous ore, preferably alumina, dissolved in molten salts, preferably a fluoride based electrolyte, in an electrolysis cell with vertical or essentially vertical electrode configuration. The invention describes methods of designing and operating inert electrodes in a vertical and/or inclined position for production of aluminum metal, where said electrodes have an operating temperature that may deviate from the electrolyte temperature, thereby controlling the dissolution of electrode materials and preventing solid deposit formation on the electrodes. The present invention is also applicable to aluminum production cells utilizing inert electrodes in a horizontal configuration, and traditional Hall-Heroult cells retrofitted with inert anodes.
US8900438 — ELECTROLYTIC CELL AND ELECTROCHEMICAL PROCESS USING AN ELECTRODE — University of Leeds (England) — The present invention relates to an electrode composed of an Al-M-Cu based alloy, to a process for preparing the Al-M-Cu based alloy, to an electrolytic cell comprising the electrode, to the use of an Al-M-Cu based alloy as an anode and to a method for extracting a reactive metal from a reactive metal-containing source using an Al-M-Cu based alloy as an anode comprising the intermetallic phase Al5M2Cu, wherein M denotes Ti and further comprises one or more metallic elements selected from the group consisting of Zr, Cr, Nb, V, Co, Ta, Fe, Ni, La and Mn, and wherein the Al-M-Cu based alloy is substantially free of CuAl2. The electrolytic cell is a Hall-Heroult cell for the extraction of aluminum.
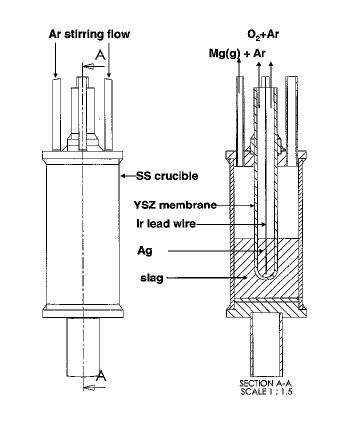
US8658007 — OXYGEN-PRODUCING INERT ANODES FOR SOM PROCESS — The Trustees of Boston University (USA) — An environmentally sound solid-oxide-membrane (SOM) electrolysis system can efficiently synthesize metals and alloys directly from their oxide ores with minimum feed-material preparation and produce oxygen gas or water vapor as the major byproduct. An electrolysis system for generating a metal and molecular oxygen includes a container for receiving a metal oxide containing a metallic species to be extracted, a cathode positioned to contact a metal oxide housed within the container; an oxygen-ion-conducting membrane positioned to contact a metal oxide housed within the container; an anode in contact with the oxygen-ion-conducting membrane and spaced apart from a metal oxide housed within the container, said anode selected from the group consisting of liquid metal silver, oxygen stable electronic oxides, oxygen stable crucible cermets, and stabilized zirconia composites with oxygen stable electronic oxides.
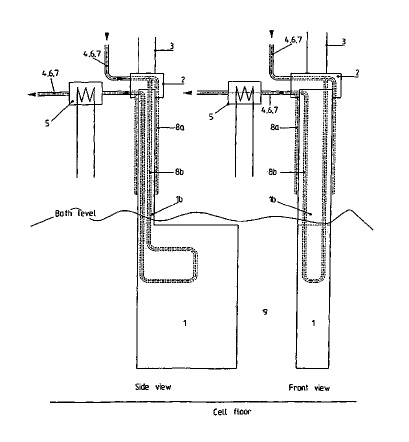
US8480876 — ALUMINUM PRODUCTION CELL — Theodore R. Beck (USA) — This invention relates to aluminum production and more particularly, it relates to smelting aluminum in a low temperature electrolytic production cell. There is provided a method of producing aluminum in an electrolytic cell containing alumina dissolved in the electrolyte, the method comprising the steps of providing a molten salt electrolyte containing an alumina slurry in an electrolytic cell having an anodic liner for containing the electrolyte, the liner having an anodic bottom and walls including at least one end wall extending upwardly from the bottom, the anodic liner being substantially inert with respect to the molten electrolyte. A plurality of non-consumable anodes is disposed substantially vertically in the electrolyte and a plurality of cathodes are is disposed vertically in the electrolyte. The anodes and cathodes are arranged in alternating relationship, and the anodes are electrically connected to the anodic liner. The cathodes have a porous, electrically conductive layer on the surface thereof wet by molten aluminum and suited to deposit aluminum thereon, the porous layer separating the molten aluminum from the electrolyte. An electric current is passed through the anodic liner to the anodes, through the electrolyte to the cathodes, and aluminum is deposited on the cathodes. Oxygen bubbles are generated at the anodes and the anodic liner, the bubbles stirring the electrolyte. Molten aluminum is collected from the cathodes in a tubular member, the tubular member in liquid communication with each cathode to collect molten aluminum therefrom. Molten aluminum is delivered by siphoning through the tubular member to a molten aluminum container.
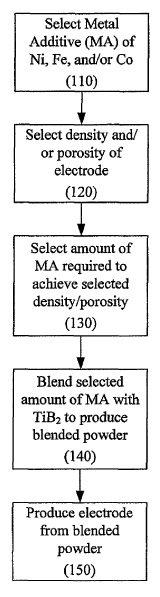
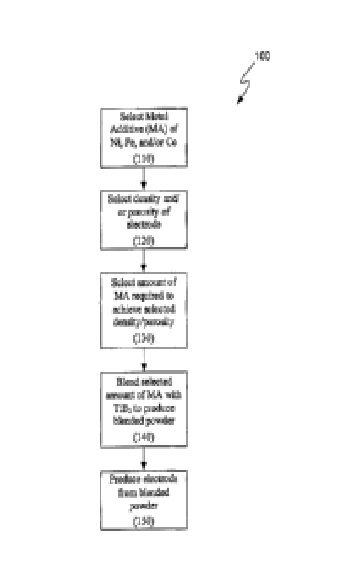
US8211278 — COMPOSITION FOR MAKING WETTABLE CATHODE IN ALUMINUM SMELTING — Alcoa Inc. (USA) — Compositions for making wettable cathodes to be used in aluminum electrolysis cells are disclosed. The compositions generally include titanium diboride (TiB2) and metal additives. The amount of selected metal additives may result in production of electrodes having a tailored density and/or porosity. An electrode for use in an aluminum electrolysis cell comprising: 0.01 to 0.75 wt. % metal additives, wherein the metal additives are selected from the group consisting of Fe, Ni, Co, and W, and combinations thereof; the balance being TiB2 and unavoidable impurities, wherein the unavoidable impurities make up less than 2 wt. % of the electrode; wherein the electrode has a density of at least about 85% of its theoretical density.
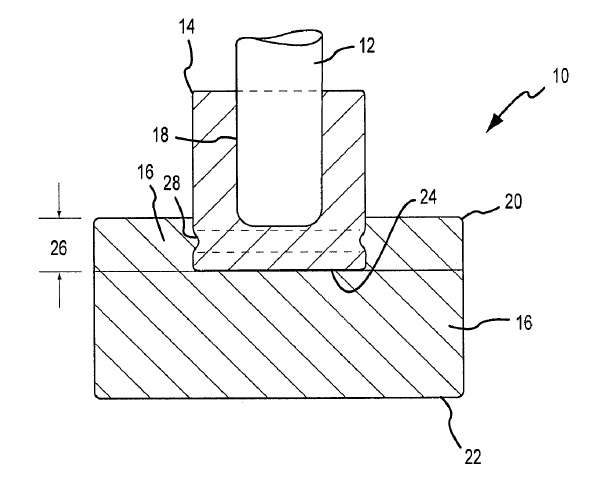
US7799182 — INERT ELECTRODE ASSEMBLIES AND METHODS OF MANUFACTURING THE SAME — Alcoa Inc. (USA) — There exists a need for larger-scale inert anode assemblies that are resistant to thermal shock and corrosion. A composite anode assembly is provided, the assembly including a permeation resistant portion and a porous conductive portion circumscribing at least the bottom of the permeation resistant portion. the permeation resistant portion is adapted to circumscribe an electrical conductor pin, the electrical conductor pin being circumscribed by a permeation resistant portion, which is circumscribed by a porous conductive portion. Methods for producing the inventive anode assemblies are also provided. One embodiment of a method useful in accordance with the present invention includes the steps of forming a permeation resistant portion, forming a porous conductive portion, and interconnecting the permeation resistant portion to the porous conductive portion. The composite anode assembly reduces corrosion and restricts thermal expansion stresses.
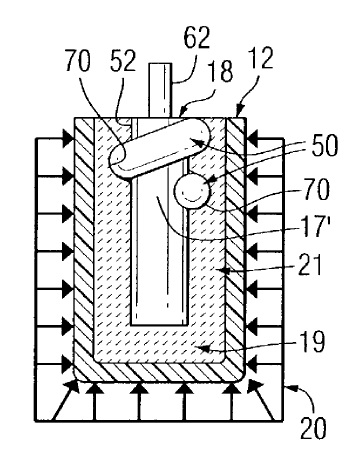
US7323134 — METHOD OF FORMING INERT ANODES — Alcoa Inc. (USA) — A method of making an inert anode (12′) for use in an electrochemical cell first provides a hollow shaped mold (12) where a metal mandrel (17′) having raised male threads (50) at its top diameter (52) is inserted into the mold (12) and a compressible powder (19, 21) added, then the powder is compressed to form recessed female grooves (70) matching the mandrel threads (50) where the mandrel (17′) is engaged and withdrawn along with the compressed powder inert anode after which the mandrel is rotated to unscrew it from the compressed powder and the compressed powder shape is then placed on a tray (27) and heated to sintering temperature.
US7316577 — NICKEL FOAM PIN CONNECTIONS FOR INERT ANODES — Alcoa Inc. (USA) — Making a low resistance electrical connection between a ceramic or ceramic-metallic inert anode and a metallic conductor has always been a challenge. The connection must be maintained with good integrity (low electrical resistance) over a wide range of temperatures and operating conditions. Also, differential thermal growth between the pin and ceramic or cermet over the assembly and process temperature range can cause the inert material to crack and/or the electrical connection to increase in resistance; rendering the assembly unfit for continued use. It is a main object of this invention to provide a low electrical resistance connection of the pin conductor and inert anode electrode. It is another object to reduce assembly costs and provide a simplified design and method. An electrode assembly useful in manufacturing aluminum, contains a hollow inert electrode (12) containing a metal conductor (14) surrounded and held in place by at least one seal (16) and a mass of metal foam (26).
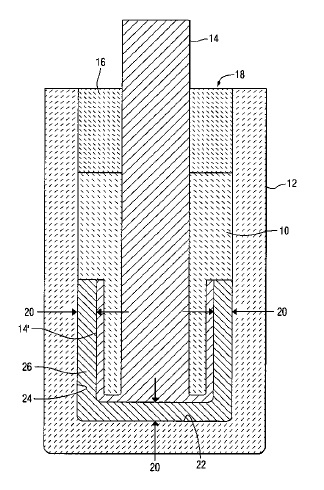
US7282133 — CERMET INERT ANODE ASSEMBLY HEAT RADIATION SHIELD — Alcoa Inc. (USA) — The present invention relates to methods for protecting operating inert anode electrodes from temperature drops upon removal and replacement of an adjacent anode. More specifically, the present invention relates to protection of inert anodes and their support structure from thermal shock when operating in a cryolite bath during adjacent anode change out operations. A method of protecting an inert anode assembly (16) operating in an electrolysis cell (10) for producing metal when an adjacent assembly (16′) is removed exposing remaining assemblies to low ambient temperatures (40) by utilizing heat radiation shields (24) which can circumscribe every inert anode assembly (16), where the shields (24) remain intact and in place in the cell (10) while operating in molten electrolyte (15) at about 850°C.
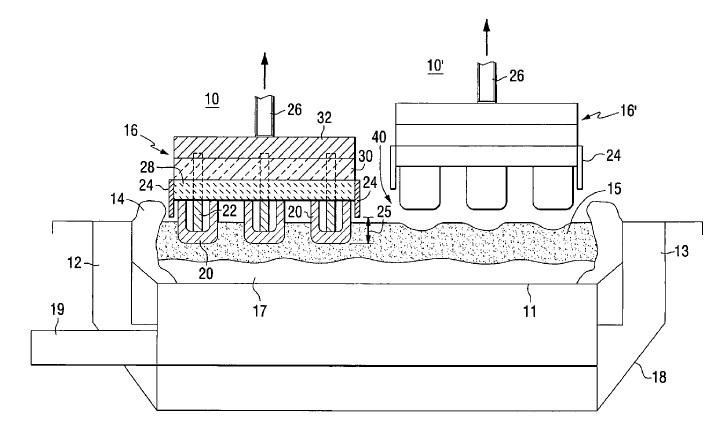
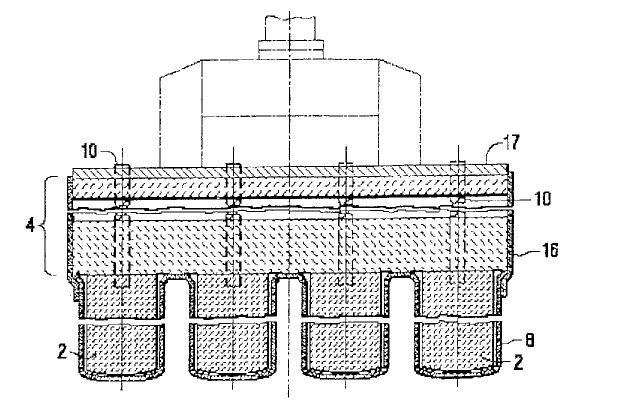
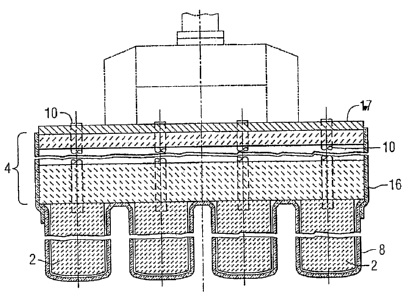
US7118666 — PROTECTING AN INERT ANODE FROM THERMAL SHOCK — Alcoa Inc. (USA) — The present invention is directed to a method for protecting ceramic or cermet inert anodes from thermal shock. The methods generally comprise applying a thermal insulating layer, or “boot” around the inert anode. The insulating layer or boot is applied to the anode before preheating begins and remains on the anode during positioning of the anode into the cell and submersion of the anode into the molten bath. A method for protecting ceramic or cermet anode assemblies in an electrolytic cell from thermal shock is disclosed. The method generally involves applying a thermal insulating layer (8, 16) to the anode (2) prior to preheating the anode assembly in a furnace, where the layer (8, 16) protects the anode (2) from thermal shock during transfer from the preheat furnace to the electrolytic cell. In a preferred embodiment the anode (2) is attached to a castable plate (4) that is also protected from thermal shock by an insulating layer.
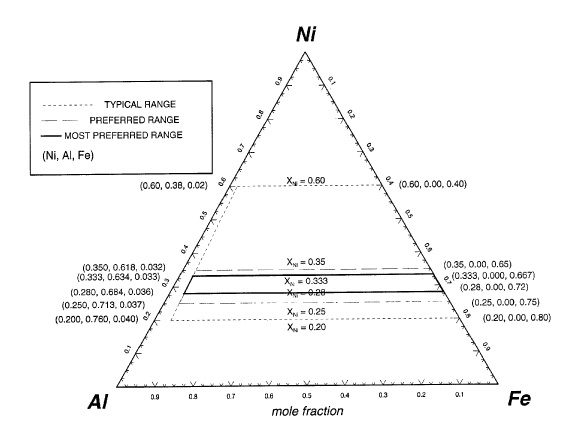
US7033469 — STABLE INERT ANODES INCLUDING AN OXIDE OF NICKEL, IRON AND ALUMINUM — Alcoa Inc. (USA) — Ceramic inert anodes useful for the electrolytic production of aluminum are disclosed. The inert anodes comprise oxides of Ni, Fe and Al. The Ni-Fe-Al oxide inert anode materials have sufficient electrical conductivity at operation temperatures of aluminum production cells, and also possess good mechanical stability. In one embodiment, the Ni-Fe-Al oxide may consist essentially of a single phase at an operation temperature of the electrolytic aluminum production cell. The Ni-Fe-Al oxide inert anodes may be used to produce commercial purity aluminum.
US6821312 — CERMET INERT ANODE MATERIALS AND METHOD OF MAKING SAME — Alcoa Inc. (USA) — A method of making cermet inert anodes for the electrolytic production of metals such as aluminum is disclosed. The method includes the step of spray drying a slurry comprising ceramic phase particles and metal phase particles. The resultant spray dried powder, which comprises agglomerates of both the ceramic phase and metal phase particles, may then be consolidated by techniques such as pressing and sintering to produce a cermet inert anode material. The ceramic phase may comprise oxides of Ni, Fe and at least one additional metal selected from Zn, Co, Al, Li, Cu, Ti, V, Cr, Zr, Nb, Ta, W, Mo, Hf and rare earths. The metal phase may comprise Cu, Ag, Pd, Pt, Au, Rh, Ru, Ir and/or Os. The consolidated cermet inert anode material exhibits improved properties such as reduced porosity. The cermet inert anodes may be used in electrolytic reduction cells for production of commercial purity aluminum as well as other metals.
CA3037199 — ANODE APPARATUS AND METHODS REGARDING THE SAME — Elysis Limited Partnership (Canada) — In some embodiments, an inert anode apparatus comprises: (a) an anode body comprising at least one outer sidewall, wherein the outer sidewall is configured to define a shape of the anode body, and to perimetrically surround a hole in the anode body, wherein the hole comprises an upper opening in a top surface of the anode body and wherein the hole axially extends into the anode body; (b) a pin comprising: a first end and a second end opposite the first end, wherein the second end extends downward into the upper end of the anode body and into the hole of the anode body; and (c) a sealing material configured to cover at least a portion of at least one of the following: (1) an inner sidewall of the anode body; (2) the top surface of the anode body; (3) the pin; and (4) the anode support.
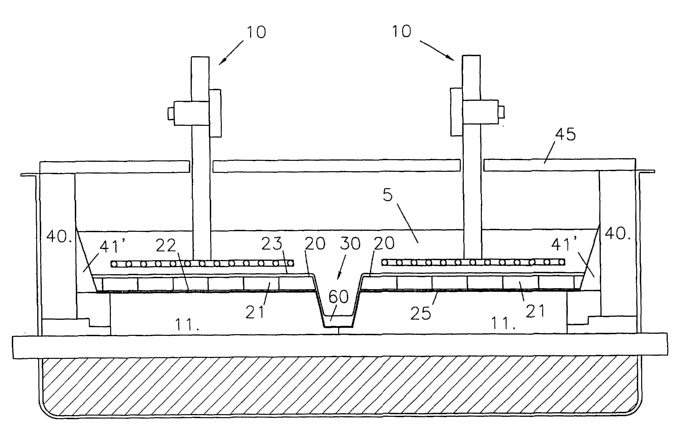
CA3030330 — ADVANCED ALUMINUM ELECTROLYSIS CELL — Elysis Limited Partnership (Canada) — In some embodiments, an electrolytic cell includes: an one anode module having a plurality of anodes; a one cathode module, opposing the anode module, and comprising a plurality of vertical cathodes, wherein each of the plurality of anodes is an oxygen evolving electrode and each of the plurality of vertical cathodes are vertically oriented and spaced one from another; a cell reservoir; and a cell bottom supporting the cathode module, wherein the cell bottom comprise an first upper surface, a second upper surface, and a channel, wherein the plurality of vertical cathodes extends upward from the upper surfaces, wherein at least one cathode block is located below the plurality of vertical cathodes, wherein the first upper surface and the second upper surface are configured to direct substantially all of the liquid aluminum produced in the electrolytic cell to the channel, and wherein the channel is configured to receive liquid aluminum from the upper surfaces.
CA2983583 — ALUMINUM REDUCTION CELL ELECTRODE (VARIANTS) — Obshchestvo S Ogranichennoy Otvetstvennost’yu “Obedinennaya Kompaniya Rusal Inzhenerno-Tekhnologicheskiy Tsentr” (Russia) — The invention relates to vertical or inclined electrodes of a reduction cell for electrolytically producing aluminum from aluminum oxide. An electrode contains an electrode base and a surface coating based on refractory ceramics. According to a first variant of the invention, the electrode base is made of a composite material containing between 5% and 90% by mass of refractory ceramics, and of at least one metal having a melting temperature exceeding 1000 °C, which forms refractory intermetallic compounds upon interaction with aluminum, and/or containing at least one alloy of such a metal. According to a second variant of the invention, the electrode base is made of a metal alloy, for example structural steel or another alloy, and the surface of the electrode base has applied thereto an intermediary layer consisting of a composite material having the composition described above.
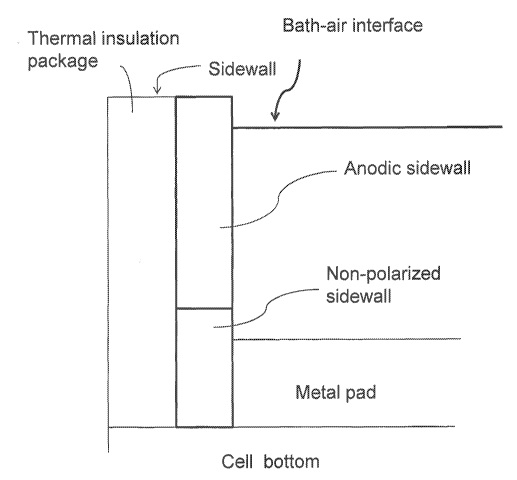
CA2960605 — SYSTEMS AND METHODS OF PROTECTING ELECTROLYSIS CELL SIDEWALLS — Elysis Limited Partnership (Canada) — Traditionally, sidewalls of an electrolysis cell are constructed of thermally conductive materials to form a frozen ledge along the entire sidewall (and upper surface of the bath) to maintain cell integrity. Broadly, the present disclosure relates to sidewall features (e.g. inner sidewall or hot face) of an electrolysis cell, which protect the sidewall from the electrolytic bath while the cell is in operation (e.g. producing metal in the electrolytic cell). More specifically, in one or more embodiments of the instant disclosure, the inner sidewall features provide for direct contact with the metal, bath, and/or vapor in an electrolytic cell in the absence of the frozen ledge along the entire or a portion of inner sidewall. in some embodiments, a stable sidewall material is provided, which is stable (e.g, substantially non-reactive) in the molten electrolyte (e.g, the cell bath) by maintaining one or more components in the bath chemistry at a certain percentage of saturation.
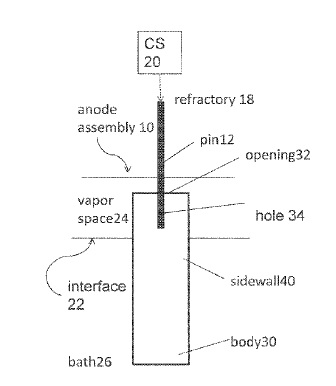
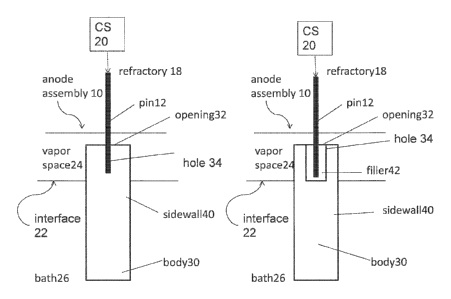
CA2960165 — ANODE APPARATUS — Elysis Limited Partnership (Canada) — Generally, the instant disclosure is directed towards an inert anode apparatus, including a pin where the pin extends into the anode body to a certain location (e.g. depth into a hole in the anode body). More specifically, the instant disclosure is directed towards an inert anode apparatus, including a pin which provides an electrical and mechanical connection to the anode body, where the pin extends into the anode body to a certain portion of the total length of the anode body, and is positioned inside the anode (e.g. in the anode hole) such that during operation of the anode (i.e. in an electrolysis cell to produce non-ferrous metal), the pin is above the bath-vapor interface and the present disclosure relates to an inert anode which is electrically connected to the electrolytic cell, such that a conductor rod is connected to the inert anode in order to supply current from a current supply to the inert anode, where the inert anode directs current into the electrolytic bath to produce non-ferrous metal (where current exits the cell via a cathode).
CA2917436 — IRON-BASED ANODE FOR OBTAINING ALUMINUM BY THE ELECTROLYSIS OF MELTS — Obshchestvo s Ogranichennoy Otvetstvennost’yu “Obedinennaya Kompaniya Rusal Inzhenerno-Tekhnologicheskiy Tsentr” (Russia) — The invention concerns non-ferrous metallurgy, particularly an anode for the electrolytically obtaining aluminum by the electrolysis of fluoride melts. The anode for obtaining aluminum by means of the electrolysis of melts at a temperature of less than 930°C consists of a base executed of a alloy containing 65-96%wt of iron, up to 35%wt of copper, up to 20%wt of nickel, and one or several additives from molybdenum, manganese, titanium, tantalum, tungsten, vanadium, zirconium, niobium, chromium, aluminum (up to 1%wt), cobalt, cerium, yttrium, silicon, and carbon totaling up to 5%wt, and a protective oxide layer consisting mainly of iron oxides and complex oxides of iron, copper, and nickel. The base is made by casting in a metal or sand mold. The protective oxide layer on the anode surface is obtaining by preliminary oxidation in air at a temperature of 850-1050°C or subsequently in the electrolysis process by oxidation with oxygen evolving at the anode. The protective oxide layer at the anode surface has a thickness of 0.1-3.0 mm.
CA2917342 — ELECTROLYTE FOR OBTAINING MELTS USING AN ALUMINUM ELECTROLYZER — Obshchestvo s Ogranichennoy Otvetstvennost’yu “Obedinennaya Kompaniya Rusal Inzhenerno-Tekhnologicheskiy Tsentr” (Russia) — The invention concerns non-ferrous metallurgy, particularly the composition of an electrolyte for electrically obtaining aluminum by the electrolysis of fluoride melts. The electrolyte proposed contains, in % by weight: sodium fluoride 26-43, potassium fluoride up to 12, lithium fluoride up to 5, calcium fluoride 2-6, alumina 2-6, aluminum fluoride and admixtures the remainder. The technical result is to increase the solubility of alumina in the electrolyte at a temperature of 830-930°C. In the electrolyte being applied for, the carbon and inert electrode materials are not destroyed, and the use of special methods to purify the aluminum of melt components is not required.
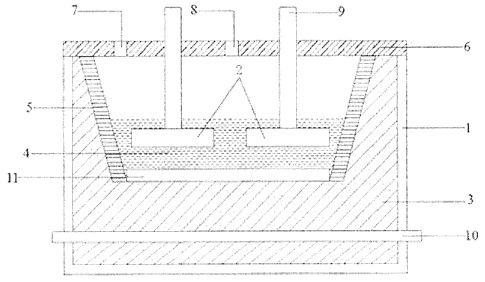
CA2900418 — CATHODE BLOCK HAVING AN ABRASION-RESISTANT SURFACE THAT CAN BE WETTED — SGL CFL CE GMBH (Germany) — The object of the present invention is therefore to provide a cathode block which is suitable in particular for use for an aluminum electrolysis cell and which not only has a low electrical resistivity and is highly wettable with aluminum melt, but is also distinguished in particular by a high wear-resistance towards the abrasive chemical and thermal conditions prevailing during the operation during melt-flow electrolysis, in particular including at high current intensities of for example 600 kA. The invention relates to a cathode block for an aluminum electrolytic cell composed, at least in some sections, of a material that can be obtained by burning a mixture, which mixture contains at least one material containing carbon having a degree of graphitization calculated in accordance with Maire and Mehring from the mean layer distance c/2 after a heat treatment at 2,800°C of at most 0.50 and at least one non-oxide ceramic.

CA2891214 — ALUMINUM ELECTROLYSIS CELL CATHODE SHUNT DESIGN — Obshchestvo s Ogranichennoy Otvetstvennost’yu “Obedinennaya Kompaniya Rusal Inzhenerno-Tekhnologicheskiy Tsentr” (Russia) — The invention relates to non-ferrous metallurgy, particularly, electrowinning of aluminum from cryolite-alumina melts, and can be used in the shunt design of a cathode assembly. In an aluminum electrolysis cell, cathode vertical metal shunts, which conduct electrical current from the aluminum melt to the cathode busbar, are designed such that their top part is melted aluminum, and the bottom part is solid aluminum. Shunts are located in conduits made in a hearth slab lining which has a widening in the middle part which is wider than both parts of the shunts. The widening in the shunt conduit can be filled with a composite material, i.e. titanium diboride-carbon. The shunts can be designed as a tube, and the widening in the conduit and the space inside the tube can be filled with the composite material titanium diboride-carbon. The application of the proposed technical solution makes it possible to significantly increase the electrical efficiency due to the absence of contact assemblies which contain dissimilar materials in the cathode shunt, due to reduced current loss, and due to achieving a guaranteed effective current distribution and an effective current shunting.
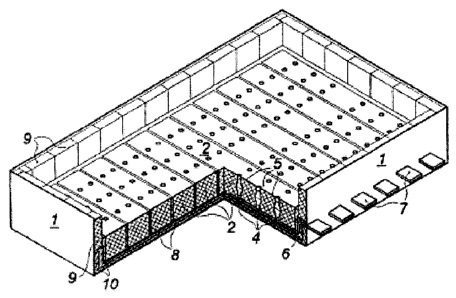
CA2877591 — ELECTROLYTIC CELL FOR ALUMINUM ELECTROLYSIS AND ELECTROLYSIS PROCESS USING ELECTROLYTIC CELL – Inner Mongolia United Industrial Co., Ltd. (China) — An electrolysis tank used for aluminum electrolysis, comprising a tank body, an anode and a cathode arranged within the tank body, also an electrolyte accommodated within the tank body, where at least a part of the anode is submerged in the electrolyte. The anode is arranged above the tank body. The cathode is arranged at the tank bottom and is covered by a certain amount of liquid aluminum. The electrolyte is provided between the anode and the cathode. The electrolyte covers the liquid aluminum. The tank body has arranged on an inner sidewall thereof an insulation layer for use in separating oxygen or the electrolyte from a carbon block. The tank is characterized in that: the constituents of the anode comprise Fe, Cu, Ni, and Sn, where Fe and Cu are the main constituents; the electrolyte consists of 30 to 38 wt% of NaF, 49 to 60 wt% of AlF3, 1 to 5 wt% of LiF, 1 to 6 wt% of KF, and 3 to 6 wt% of Al2O3, where the molar ratio of NaF to AlF3 is between 1.0 and 1.52. The electrolysis tank is applicable in industrialized production of electrolyzed aluminum.
CA2876336 — INERT ALLOY ANODE FOR ALUMINUM ELECTROLYSIS AND PREPARING METHOD THEREOF — Inner Mongolia United Industrial Co., Ltd. (China) — An inert alloy anode used for aluminum electrolysis has Fe and Cu as the main constituents and comprises Sn. The addition of the Sn metal is conducive to the formation of a layer of oxidized film having a great antioxidant activity and structural stability on the surface of the inert alloy anode and is conducive to an increase in the corrosion resistance of the anode. On this basis, the constituents of the inert alloy anode also comprise Ni, Al, and Y. The addition of the Al metal prevents the main metal constituents from being oxidized, the addition of the Y metal controls the alloy to provide a required polymorph in a preparation process, thus achieving the goal of anti-oxidation. The inert alloy anode having Fe and Cu as the main constituents has a low over-voltage, high electric conductivity, and reduced costs, and is applicable in the aluminum electrolysis industry.
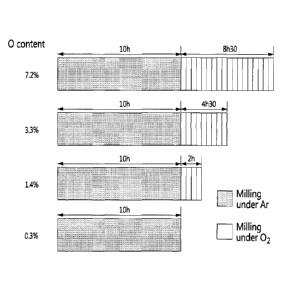
CA2850856 — INERT ANODES FOR ALUMINUM ELECTROLYSIS AND METHOD OF PRODUCTION THEREOF — Institut National de la Recherche Scientifique (Canada) — An inert anode for Al electrolysis, made of Cu-Ni-Fe-0 based materials, comprising Fe in a range between about 10 and 20 % by weight, Cu in a range between about 60 and about 80% by weight, Ni in a range between about 20 and about 30% by weight, and oxygen in a range between about 1 and about 3% by weight, and a method for producing the anode, comprising mechanically alloying metallic elements; oxygen doping; and consolidation.
CA2826604 — CATHODE BLOCK HAVING A TOP LAYER CONTAINING HARD MATERIAL — SGL CFL CE GMBH (Germany) — A cathode block for an aluminum electrolytic cell comprises a primary layer and a cover layer arranged thereon, the primary layer containing graphite and the cover layer being composed of a carbon composite material which contains 15 to less than 50% by weight of a hard material having a melting point of at least 1.000°C. Examples of suitable hard materials are metal carbides, metal borides, metal nitrides and metal carbonitrides having a sufficiently high hardness at 1000°C.
CA2787207 — ELECTROLYTIC CELL FOR PRODUCING PRIMARY ALUMINUM BY USING INERT ANODE — Aluminum Corporation of China Limited (China) — An electrolytic cell for producing primary aluminum by using inert anodes is disclosed, in which an electrolyte system KF-NaF-AlF3 is used and the operating temperature of the cell is 700-850. The electrolytic cell comprises cell shell, heat insulating refractory lining, melting pot, heat insulating cover, inert anodes, electrode stem, anode bus-bars, cathode bus-bars, anode branching bus-bars, heat insulating plate, partitions between anodes and cathodes and feeding device. The quality of the aluminum product obtained by using the electrolytic cell is not less than 99.7%. The cell is free from emission of carbon dioxide and perfluorinated compounds (PFCs), and hardly has consumption of electrodes, so the distances between the anodes and cathodes can be kept stable. The cell is sealed and the volatilization of dust and fluorides can be prevented, and it is useful to recover oxygen gas. The electrolytic cell has good heat insulation effect, increased heat efficiency, reduced heat loss, increased space utilization rate and capacity in unit volume or unit floor space of the cell. The electrolytic cell is free of tank leakage, and it has long service life and flexible design for cell structure.
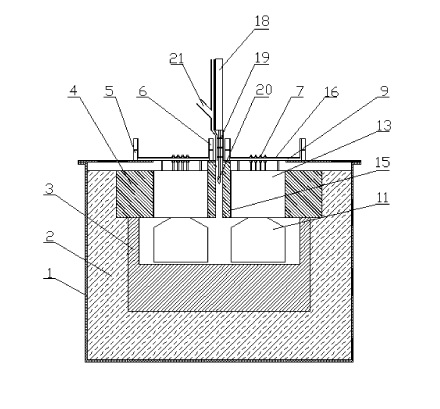
CA2775096 — INERT ANODE ASSEMBLY — Elysis Limited Partnership (Canada) — The present invention relates to structures and methods for protecting inert anodes and other electrodes and electrode support materials from degradation by a cryolite-based molten electrolyte bath, and from HF/02 and other gases generated in an electrolytic cell. The present invention also improves metal production, such as aluminum production, by limiting bath and metal contamination and reducing thermal shock during initial preheating and placement of anodes in electrolytic cells. A solid material (12′) circumscribing an anode system (10) in an electrolysis apparatus is made from a mixture of cryolite and/or alumina (Al2O3), where the solid material (12′) contacts and surrounds the anodes (14, 14′).

CA2768992 — COMPOSITION FOR MAKING WETTABLE CATHODE IN ALUMINUM SMELTING — Alcoa Inc. (USA) — Compositions for making wettable cathodes to be used in aluminum electrolysis cells are disclosed. The compositions generally include titanium diboride (TiB2) and metal additives. The amount of selected metal additives may result in production of electrodes having a tailored density and/or porosity. The electrodes may be durable and used in aluminum electrolysis cells. ne embodiment discloses a composition generally comprising titanium diboride (TiB2). In some embodiments, a composition consists essentially of titanium diboride and at least one metal additive, the balance being unavoidable impurities. In some embodiments, the metal additive includes Co, Fe, Ni, and W, among others.
CA2753404 — PROTECTING AN INERT ANODE FROM THERMAL SHOCK — Elysis Limited Partnership (Canada) — A method for protecting anode assemblies in an electrolytic cell from thermal shock is disclosed. The method generally involves applying a thermal insulating layer (8, 16) to the anode (2) prior to preheating the anode assembly in a furnace, where the layer (8, 16) protects the anode (2) from thermal shock during transfer from the preheat furnace to the electrolytic cell. In a preferred embodiment the anode (2) is attached to a castable plate (4) that is also protected from thermal shock by an insulating layer.
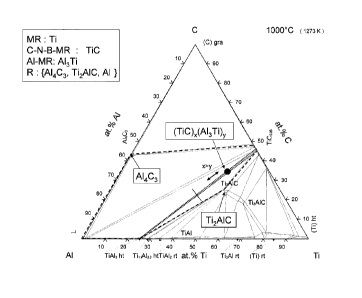
CA2739281 — COMPOSITE MATERIALS FOR WETTABLE CATHODES AND USE THEREOF FOR ALUMINIUM PRODUCTION — Hydro-Quebec (Canada) — The invention relates to a composite material of formula: (C-N-B-MR)x(Al-MR)y(R)z in which C-N-B-MR is one or more carbides, nitrides or borides of one or more refractory metals of group IV, V or VI of the Periodic Table and/or one or more aluminum carbides, nitrides or borides chosen from Al4C3, AlN, AlB2 and Al1-67B22; Al-MR is one or more aluminides of one or more of the above refractory metals, it being understood that: if MR = Nb, Ta, Hf, Zr, Ti or V, then Al-MR = Al3MR; if MR = W or Cr, then Al-MR = Al4MR; if MR = Mo, then Al-MR = Al8Mo3 or Al 17Mo4 (~Al4Mo), and R is a residual component other than 4 carbon, comprising one or more phases chosen from Al4C3, AlN, AlB2, Al1-67B2 and MRtAlu(C-N-B)v in which t, u and v are numbers greater than or equal to zero, and x, y and z are the volume fractions of the respective components with x > y; x + y > 0.5; x + y + z = 1 and 0.01 < y < 0.5. The invention is also aimed at the use of this composite material as a coating in a component that can be wetted by liquid aluminum and can be used in electrolysis cells.
CA2737182 — NOVEL COMBINED GRAPHITIZED IRREGULAR CATHODE FOR ALUMINUM AND GRAPHITIZED CATHODE STOP BLOCK THEREOF — Guangxi Qiangqiang Carbon Co. Ltd. (China) — A novel combined graphitized irregular cathode for aluminum comprises body blocks and graphitized cathode stop blocks; the graphitized cathode stop blocks are prepared from raw materials including: calcined petroleum coke, electrically calcined anthracite, coal pitch, TiB2 alloy additive and SiC additive. According to the invention, the graphitized cathode stop blocks are inlaid at a junction seam of two cathode body blocks in a manner of bridging over the cathode, thus electrode distance of aluminum electrolysis is shortened, cell voltage is lowered by about 0.35 volts to 0.5 volts; the energy per ton aluminum can be saved not less than 1000KWh to achieve prominent effect of energy-saving and consumption-reduction. The invention can avoid grooving in the middle of the body blocks, reduces the effective thickness of body blocks to impact on life-span, and implements reinforcement of local structure on the junction seam of body blocks to prolong life-span of electrolytic cell.

CA2684041 — ALUMINIUM ELECTROWINNING CELL WITH METAL-BASED CATHODES — Rio Tinto Alcan International Limited/Rio Tinto Alcan International Limitee (Canada) — A cell for the electrowinning of aluminum has one or more anodes (10) facing at least one cathode (20). This cathode comprises: a cathode body (25) made predominantly of at least one hard metal selected from tungsten and molybdenum; and a surface of carbide (26,27) of this hard metal which is integral with the body or which is formed by a layer bonded to the body. The carbide surface forms a cathodic operative surface on which during use aluminum (9) is produced or forms an anchorage surface for an aluminum-wettable ceramic layer (28,29) on which during use aluminum is produced.

CA2539697 — DEVICE AND METHOD FOR CONNECTING INERT ANODES FOR THE PRODUCTION OF ALUMINIUM BY FUSED-SALT ELECTROLYSIS — Aluminium Pechiney (France) — The invention relates to an anode assembly (1) for a cell used to produce aluminum by fused-salt electrolysis. The assembly comprises an inert anode (2) in the form of a pocket, a connecting conductor (3, 4, 5), mechanical connection means which can cooperate in order to establish a mechanical link between the conductor and the anode, and a soldered metal joint or a metal joint which can be formed by soldering (31) and which is arranged either between all or part of at least one surface (20, 20, 20′) of the open extremity (22) of the anode (2) and all or part of at least one surface (40, 40′, 40″) of the extremity of the connection (42) of the conductor (3, 4, 5). The invention makes it possible to simplify the production of anode assemblies comprising an inert anode.
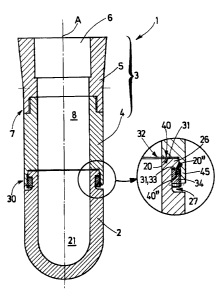
CA2519170 — MECHANICAL ATTACHMENT OF ELECTRICAL CURRENT CONDUCTOR TO INERT ANODES — Elysis Limited Partnership (Canada) — An inert anode (10) for use in an electrolysis process to make metals such as aluminum, contains a hollow interior with an open top portion (16), an interior closed bottom (18) and interior sidewalls (19) where the top interior sidewalls (16) have at least one interior groove (20) which helps relieve stress on the anode material and helps provide locking and support of the anode.
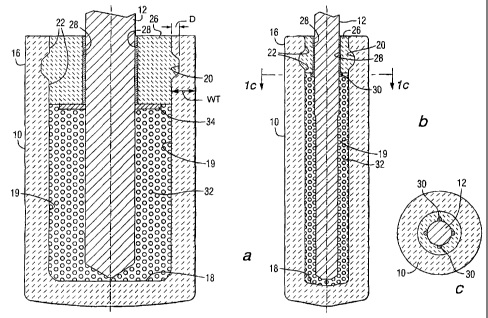
CA2519054 — RECOVERY OF METAL VALUES FROM CERMET — World Resources Company (USA) — Cermet material obtained from unused inert anodes, used inert anodes and cermet used in the production of inert anodes is beneficiated into a nonferrous metal concentrate composition from which metal values contained in the composition may be readily recovered by using a conventional smelting process. The invention also relates to the use of the composition in a smelting process for the recovery of metal values from the cermet composition of the invention.
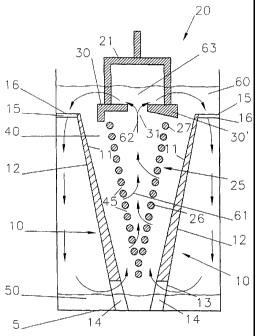
CA2506219 — INERT ANODE ASSEMBLY — Elysis Limited Partnership (Canada) — The present invention relates to structures and methods for protecting inert anodes and other electrodes and electrode support materials from degradation by a cryolite-based molten electrolyte bath, and from HF and other gases generated in an electrolytic cell. The present invention also improves metal production, such as aluminum production, by limiting bath and metal contamination and reducing thermal shock during initial preheating and placement of anodes in electrolytic cells. A solid material (12′) circumscribing an anode system (10) in an electrolysis apparatus is made from a mixture of cryolite and/or alumina (A12O3), where the solid material (12′) contacts and surrounds the anodes (14, 14′).
CA2464406 — A DIMENSIONALLY STABLE ANODE FOR THE ELECTROWINNING OF ALUMINUM — Norsk Hydro ASA (Norway) — An anode for the electrolysis of aluminum made from an outer dense layer of a ceramic material on a dense core made from a composite of the ceramic material of the outer layer and an electronic conductor. An anode for the electrolysis of aluminum made from an outer layer of a ceramic material with essentially zero open porosity on a core made from a composite of the ceramic material of the outer layer and an electronic conductor made of a metal or metal alloy forming a continuous network.
CA2459010 — ALUMINUM ELECTROWINNING CELLS WITH INCLINED CATHODES — Elysis Limited Partnership (Canada) — A cell for the electrowinning of aluminum (50) from alumina dissolved in a molten electrolyte comprises a generally horizontal cell bottom (5), preferably aluminum-wettable, on which a pool of product aluminum (50) is collected from at least one electrically conductive cathodic element (10) having aluminum-wettable cathode surfaces (11). The cathodic element comprises an inclined cathodic wall (10) in the electrolyte (60) above the generally horizontal cell bottom (5). The cathodic wall (10) has an upwardly-oriented inclined face (11) that forms a sloping upper aluminum-wettable drained active cathode surface on which aluminum is produced and drains into the aluminum pool (50), and a downwardly-oriented inclined face (12) which is in contact with the molten electrolyte (60) and which overlies the aluminum pool (50). The aluminum pool (50) covers substantially the entire cell bottom (5) including underneath the cathodic wall (10). A return path for alumina-enriched electrolyte (60) towards a bottom end of the anode-cathode gap (40) may be provided behind the cathodic wall (10) along an inactive surface (12) thereof. The cell may be fitted with anodes (10) that are foraminate, e.g. an arrangement of spaced apart parallel rods, or solid plates.
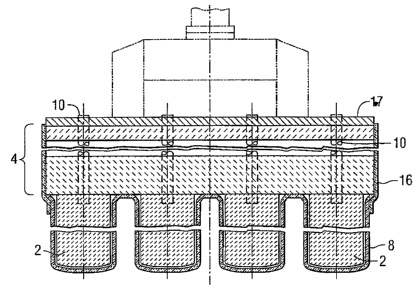
CA2458692 — PROTECTING AN INERT ANODE FROM THERMAL SHOCK — Elysis Limited Partnership (Canada) — A method for protecting anode assemblies in an electrolytic cell from thermal shock is disclosed. The method generally involves applying a thermal insulating layer (8, 16) to the anode (2) prior to preheating the anode assembly in a furnace, where the layer (8, 16) protects the anode (2) from thermal shock during transfer from the preheat furnace to the electrolytic cell. In a preferred embodiment the anode (2) is attached to a castable plate (4) that is also protected from thermal shock by an insulating layer.
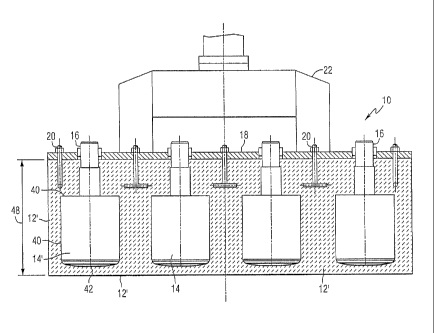
CA2448313 — ALUMINIUM ELECTROWINNING CELLS HAVING A DRAINED CATHODE BOTTOM AND AN ALUMINIUM COLLECTION RESERVOIR — Moltech Invent S.A. (Luxembourg) — A cell for the electrowinning of aluminum has a cell bottom that comprises: a series of carbon cathode blocks (25) having an aluminum-wettable upper surface (22); aluminum-wettable openly porous plates (21) which are placed on the upper surfaces (22) of the carbon cathode blocks (25) and which are filled with molten aluminum to form an aluminum-wetted drained active cathode surface (20) above the upper surfaces (22) of the carbon cathode blocks (25); and a recess (35) formed in a reservoir body 30 and located at a level below the upper surfaces (22) of the carbon cathode blocks (25) and which during use collects molten aluminum (60) drained from the aluminum-wettable drained active cathode surface (20). Openly porous plates (21) on the upper surfaces (22) of the carbon cathode blocks (25) extend over at least part of the recess (35) such that part of the aluminum-wetted drained active cathode surface (20) is located over the aluminum collection recess (35).
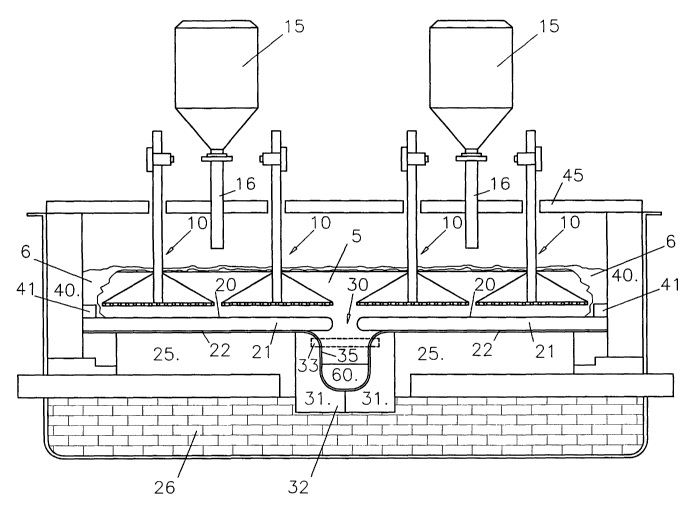
CA2439011 — A METHOD AND AN ELECTROWINNING CELL FOR PRODUCTION OF METAL — Norsk Hydro ASA (Norway) — The present invention relates to a method and an electrowinning cell for the production of aluminum, e.g., electrowinning of aluminum by the use of substantially inert electrodes. The present invention relates to a method for production of molten aluminum by electrolysis of an aluminous ore, preferably alumina, in a molten salt mixture, preferably a sodium fluoride – aluminum fluoride-based electrolyte. The invention describes an electrolysis cell for said production of aluminum by use of essentially inert electrodes in a vertical and/or inclined position, where said cell design facilitates separation of aluminum and evolved oxygen gas by providing a gas separation chamber (14) arranged in communication with the electrolysis chamber (22), thus establishing an electrolyte flow between the electrolysis chamber (22) and the gas separation chamber (14).
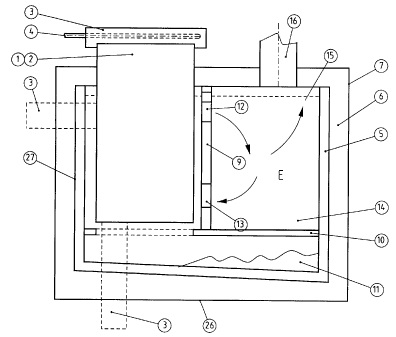
CA2439006 — A MATERIAL FOR A DIMENSIONALLY STABLE ANODE FOR THE ELECTROWINNING OF ALUMINIUM — Norsk Hydro ASA (Norway) — A material suitable for use as the active anode surface in the electrolytic reduction of alumina to aluminum metal defined by the formula: A1+xB1+δCdO4 where A is a divalent cation or a mixture of cations with a relative preference for octahedral coordination, B is a trivalent cation or mixture of cations with a relative preference for tetrahedral coordination, C is a trivalent cations with a relative preference for octahedral coordination or a four-valent cation with a relative preference for octahedral coordination, O is the element oxygen. When C is trivalent: x=0, 0.8<d<1, δ<0.2, and x + d + δ ≈ 1.
CA2438526 — ALUMINUM-WETTABLE POROUS CERAMIC MATERIAL — Moltech Invent S.A. (Luxembourg) — A material, for instance used as an aluminum-wettable component (21,21′, 41,41′, 51), in particular of a cell for the electrowinning of aluminum (60), comprises an openly porous or reticulated ceramic structure whose surface during use is exposed to and wetted by molten aluminum. The structure is made of ceramic material inert and resistant to molten aluminum, such as alumina, and an aluminum-wettable material that comprises metal oxide and/or partly oxidised metal, in particular of manganese, iron, cobalt, nickel, copper or zinc, which is/are reactable with molten aluminum to form a surface layer containing alumina, aluminum and metal derived from the metal oxide and/or partly oxidised metal. The ceramic structure comprises a coating of the aluminum-wettable material on the inert and resistant ceramic material, or is made of a mixture of the inert and resistant material and of the aluminum-wettable ceramic material. The aluminum-wetted component is suitable for use as a cathode (21,21′), as a sidewall (41,41′) or as another component (51) which during use is exposed to molten aluminum (60) and/or electrolyte (5), or another oxidizing and/or corrosive media at high temperature.
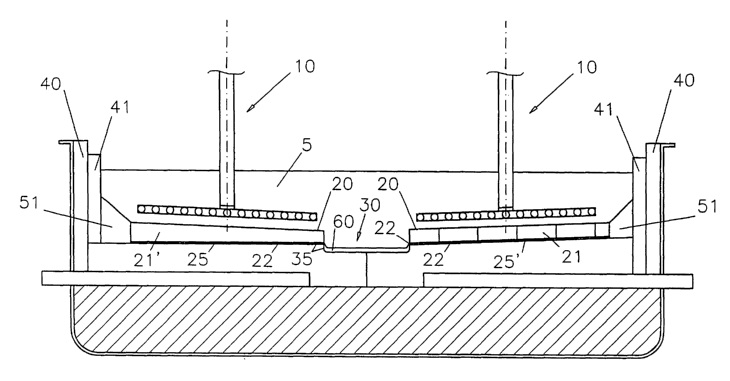
CA2437687 — CELL FOR THE ELECTROWINNING OF ALUMINUM OPERATING WITH METAL-BASED ANODES — Moltech Invent S.A. (Luxembourg) — A cell for the electrowinning of aluminum comprises a horizontal carbon cathode bottom (11) having an aluminum-wettable surface coating (25) and a series of plates (21) made of aluminum-wettable reticulated porous material, typically foams, filled with aluminum and placed flat on the aluminum-wettable surface coating (25). During use, a thin bottom layer of aluminum (22) wets the aluminum-wettable surface coating (25) on top of the cathode bottom (11) and a bottom part of the porous aluminum-filled plates (21). A top layer of aluminum (23) is formed above the porous aluminum-filled plates (21). The cell may be operated with metal anodes (10) possibly protected with a cerium oxyfluoride coating when operated above about 910~-930°C.