Editor’s Note: The lightest of all metals, lithium is a fundamental material for the production of lithium-ion rechargeable batteries (LIBs) which achieve high energy and power densities thanks to the unique atomic structure and electrochemical properties of lithium. Having been globally commercialized for some three decades, LIBs are available in various types, based on cathode chemistry and type of electrolyte. Typically, they use a flake graphite anode and a cathode made of lithium metal oxides (LMOs) such as lithium cobalt oxide (LCO), lithium-nickel-manganese-cobalt oxide (NMC), lithium-nickel-cobalt-aluminum oxide (NCA), lithium-manganese oxide (LMO), lithium-titanate oxide (LTO), or a lithium-iron phosphate (LFP).
The earliest LIBs were LCOs, which are widely used in portable electronic devices. For high performance applications, such as electric vehicles (EVs), the other types of LIBs listed are used in various configurations and packaging. In 2022, market analysis showed that globally there were over 26 million cars with LIBs on the road in 2022, with some 50% in China, 30% in Europe, 10% in the U.S., and 10% in other countries with estimates indicating some 200 million EVs will be sold by 2030.1
Most of the LIBs in circulation contain expensive heavy metals, such as cobalt, nickel, and manganese. In fact, The Wall Street Journal’s Business & Finance section regularly posts cash prices for metal commodities and includes a separate section for “Battery/EV Metals,” which lists lithium carbonate, lithium hydroxide, cobalt sulphate, nickel sulphate, and flake graphite. Both lithium carbonate and hydroxide are precursors to the lithium compounds utilized in LIBs (i.e., the cathode and the lithium salt solution used as an electrolyte), while the other LIB metal compounds are used in manufacturing the LMO cathode and flake graphite anode.
Thus, at issue here is the rapid adoption of LIBs worldwide, especially for EVs of various types, and eventually the effective disposal of the LIBs at the end of life (EOL)—not only due to their environmental pollution potential, but also due to the depletion of natural resources and the value of metal supply. In fact, a report by the U.S. Congressional Research Service (CRC) published in late 2022 identifies lithium, cobalt, manganese, nickel, and graphite as critical to meet EV battery demand domestically and globally.2
The EV battery chemistries vary, but many depend on the five critical minerals noted by the CRC, the domestic supply of which is potentially at risk for disruption. Of course, lithium’s role is supreme, without which no ionic and electron transfer would occur in LIBs.
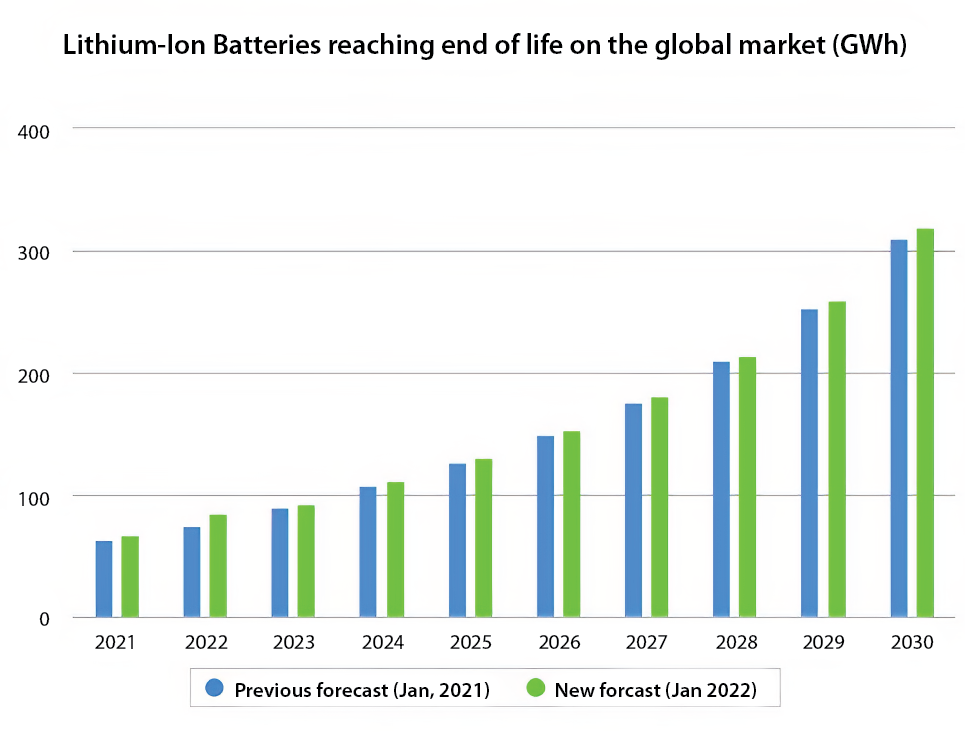
Estimates have been made about the EOL for LIBs on the global market from 2021 to 2030 (Figure 1), showing the EOL to be about 100 GWh at present and expected to grow to a little over 300 GWh (1 GWh = 1 billion Wh = 1 million kWh) in 2030. LIBs are used to power EVs of different types and sizes, ranging in capacity from 25 to 100 kWh. Therefore, if 50 kWh is taken as an average EV car battery, 100 GWh amounts to some 2 million EV batteries at EOL today.
The need for sustainable LIB recycling technology is obvious, however, a standardized LIB recycling process has not yet been established, although shredding/pretreatment is a common first step, which generates a “black mass,” essentially a cake-like material made up of LIB shredded cathodes and anodes that contain the valuable materials for further processing. Currently, further processing is done via three different LIB recycling processes:
• Hydrometallurgy – where critical materials are extracted at ordinary temperatures by leaching the “black mass” at ordinary temperatures with liquid solvents.
• Pyrometallurgy – where the battery material is smelted either with or without the shredding step and the non-metallic content is burned.
• Direct Recycling or Cathode-to-Cathode Recycling – where the valuable cathode structure is preserved, so as to reduce the amount of engineering required to cathode-active materials.
The patents selected here involve all three technologies, with a focus on inventions that advance the cost effective recovery of the critical materials in LIBs. In 2022, hydrometallurgical recycling of LIBs represented an 80.2% of the LIB recycling market share, indicating how viable this technology is at present. Overall, the global LIB recycling market size is projected to grow from $3.79 billion in 2023 to $14.89 billion by 2030, at a compound annual growth rate of 21.6%.4
However, it is also expected that inventions in new battery technology—be it LIBs or other battery technologies—will undoubtedly change how these batteries will be recycled. In general, rechargeable battery technology and invention is rapidly changing, including developments for the recycling of LIBs. As an indication, most of the U.S. patents awarded by the USPTO and selected here only date back to the past two years.
References
1. “Global EV Outlook 2023: Trends in electric light-duty vehicles,” IEA, http://www.iea.org/reports/global-ev-outlook-2023/trends-in-electric-light-duty-vehicles.
2. “Critical Minerals in Electric Vehicle Batteries,” U.S. Congressional Research Service, August 29, 2022, https://crsreports.congress.gov/product/pdf/R/R47227.
3. “Volume forecasts,” Circular Energy Storage, https://circularenergystorage.com/volumes
4. “Lithium Ion Battery Recycling Market Size… 2023-2030,” Fortune Business Insights, http://www.fortunebusinessinsights.com/industry-reports/lithium-ion-battery-recycling-market-100244.
Further Reading on the Recycling of LIBs
Several recent articles on the complexity of LIBs and their sustainability through recycling are presented here for LMA readers wishing to delve deeper into the subject, as follows:
1. Yu, M., et al., “Review: Current Li-Ion Battery Technologies in Electric Vehicles and Opportunities for Advancements,” Energies, Vol. 12, No. 6, 2019, http://www.mdpi.com/1996-1073/12/6/1074.
2. Windisch-Kern, Stefan, et al., “Recycling chains for lithium-ion batteries: A critical examination of current challenges, opportunities and process dependencies,” Waste Management, Vol. 138, 2022, pp. 125–139, https://doi.org/10.1016/j.wasman.2021.11.038.
3. Moïsé, E. and Rubinová, S., “Trade Policies to Promote the Circular Economy: A Case Study of Lithium-ion Batteries,” OECD Trade and Environment Working Papers, January 30, 2023. https://dx.doi.org/10.1787/d75a7f46-en.
4. Neumann, J., et al., “Review: Recycling of Lithium-Ion Batteries—Current State of the Art, Circular Economy, and Next Generation Recycling,” Advanced Energy Materials, Vol. 12, January 10, 2022, https://doi.org/10.1002/aenm.202102917.
5. Baum, Z. J., et al., “Lithium-Ion Battery Recycling: Overview of Techniques and Trends,” ACS Energy Letters, Vol. 7, No. 2, 2022, pp. 712–719, https://pubs.acs.org/doi/10.1021/acsenergylett.1c02602.
6. Marcinov, V., et al., “Review: Lithium Production and Recovery Methods: Overview of Lithium Losses,” Metals, Vol. 13, June 29, 2023, https://doi.org/10.3390/met13071213.
7. Agnieszka Sobianowska-Turek, et al., “Review: The Necessity of Recycling of Waste Li-Ion Batteries Used in Electric Vehicles as Objects Posing a Threat to Human Health and the Environment,” Recycling, Vol. 6, No. 35, June 1, 2021, https://doi.org/10.3390/recycling6020035.
8. Hossain, R., et al., “Technological Options and Design Evolution for Recycling Spent Lithium-Ion Batteries: Impact, Challenges, and Opportunities,” WIREs Energy and Environment, Vol. 12, May 26, 2023, https://doi.org/10.1002/wene.481.
— Joseph C. Benedyk, Editor
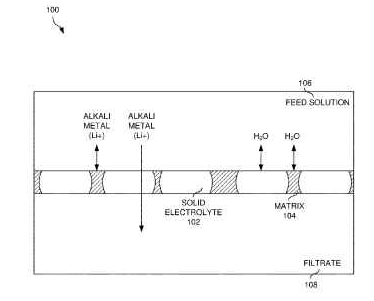
US11779886 — WATER-IMPERMEABLE CARBON-BASED ELECTROLYTE AND SEPARATION MEMBRANE AND FABRICATION THEREOF — Lyten, Inc. (USA) — The presently disclosed concepts relate to improved techniques for alkali metal extraction (in particular lithium), using a solid electrolyte membrane. The membrane may be used to extract any or all of lithium, sodium, or potassium. The solid electrolyte may include at least one of: LATP, LZP, LAGP, LiSICON, LTO, K2Fe4O7, or NaSICON. By using a solid electrolyte embedded in a matrix, alkali metal (such as lithium) can be more effectively separated from feed solutions. Additionally, energy used to initially extract lithium from a feed solution may be stored as electrochemical energy, which in turn, may be discharged when lithium is depleted from the electrode. This discharged energy may therefore be reclaimed and reused to extract additional lithium. The feed solution may include lithium ions which may be sourced from a variety of sources, including but not limited to at least one of lithium minerals, lithium-containing brines, recycled lithium batteries, or seawater.
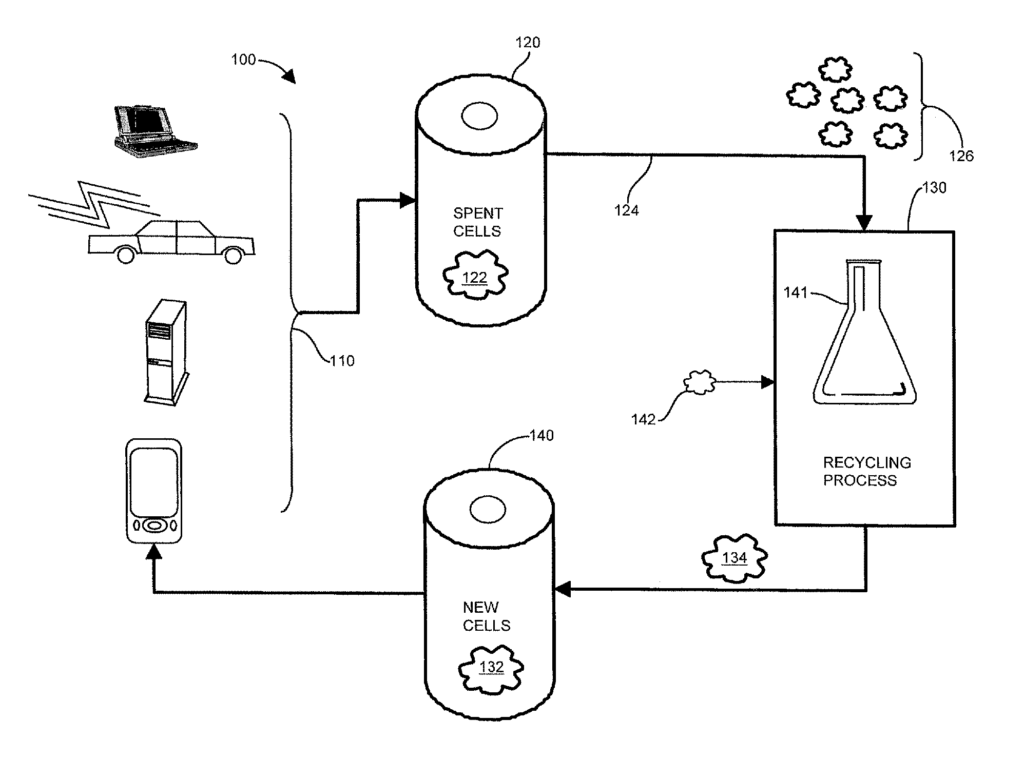
US11769916 — METHOD AND APPARATUS FOR RECYCLING LITHIUM-ION BATTERIES — Worcester Polytechnic Institute and Ascend Elements, Inc. (USA) — Cathode material from exhausted lithium-ion batteries are dissolved in a solution for extracting the useful elements Co (cobalt), Ni (nickel), Al (Aluminum) and Mn (manganese) to produce active cathode materials for new batteries. The solution includes compounds of desirable materials such as cobalt, nickel, aluminum and manganese dissolved as compounds from the exhausted cathode material of spent cells. Depending on a desired proportion, or ratio, of the desired materials, raw materials are added to the solution to achieve the desired ratio of the commingled compounds for the recycled cathode material for new cells. The desired materials precipitate out of solution without extensive heating or separation of the desired materials into individual compounds or elements. The resulting active cathode material has the predetermined ratio for use in new cells, and avoids high heat typically required to separate the useful elements because the desired materials remain commingled in solution.
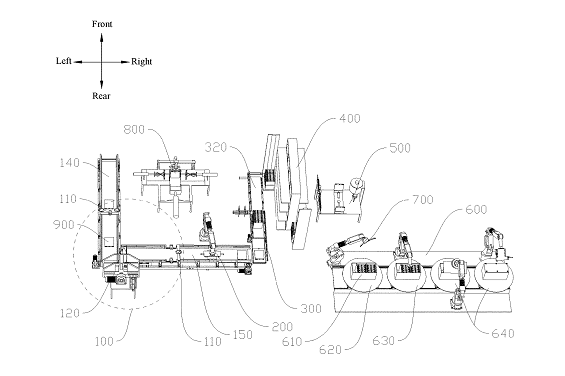
US11764390 — AUTOMATIC PRODUCTION LINE FOR CASCADE UTILIZATION OF POWER BATTERIES — Guangdong Brunp Recycling Technology Co., Ltd., Hunan Brunp Recycling Technology Co., Ltd., and Hunan, Brunp EV Recycling Co., Ltd. (China) — The present disclosure relates to the technical field of recycling retired power batteries, in particular to an automatic production line for cascade utilization of power batteries. Retired power batteries are treated with two different treatment methods according to the battery capacity: retired power batteries with a battery capacity of 20%-80% can be subjected to cascade utilization, and retired power batteries with a remaining battery level of less than 20% need to be recycled and regenerated. Disclosed is an automatic production line for cascade utilization of power batteries, which is sequentially provided along the transmission direction of materials with: an appearance detection system, a screening device, a transport system, a residual energy detection device, a tab installation device and an assembling system, and in addition, is provided with a grouping device and a film sticking device, where the grouping device is located among the residual energy detection device, the tab installation device and a grouping station.
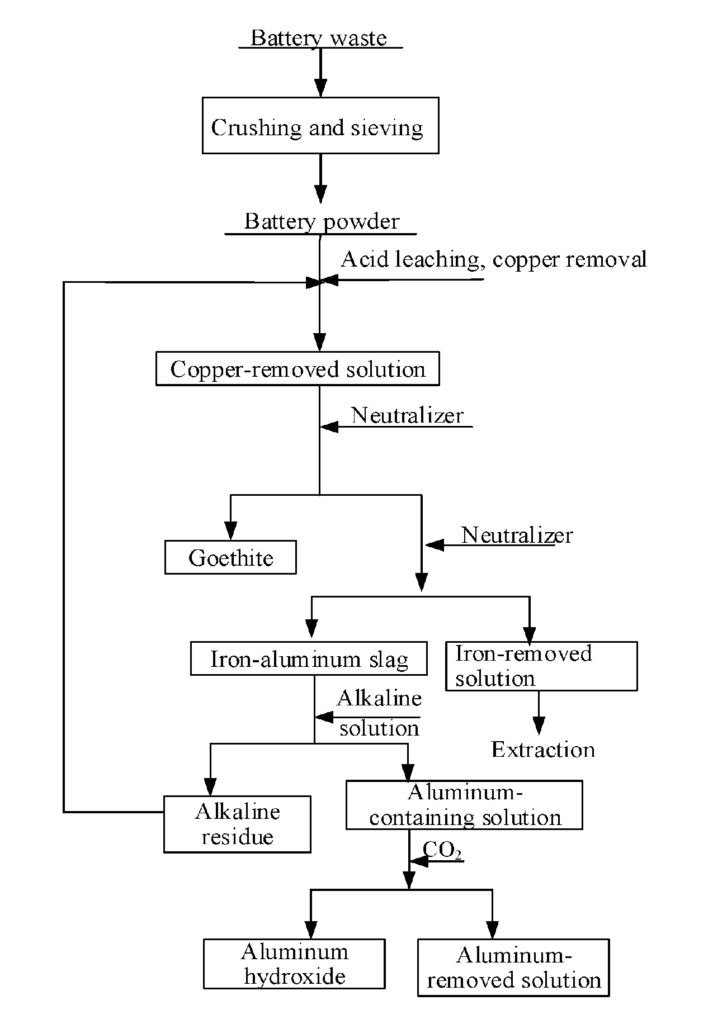
US11760655 — METHOD FOR RECYCLING IRON AND ALUMINUM IN NICKEL-COBALT-MANGANESE SOLUTION — Hunan Brunp Recycling Technology Co., Ltd., Guangdong Brunp Recycling Technology Co., Ltd., and Hunan Brunp Vehicles Recycling Co., Ltd. (China) — Iron and aluminum are main impurities during the recovery of positive electrode materials from spent lithium-ion batteries, and both the removal and recovery of the impurities have been extensively studied. The present invention relates to a method for recycling iron and aluminum in a nickel-cobalt-manganese solution. The method comprises the following steps: leaching a battery powder and removing copper therefrom to obtain a copper-removed solution, and adjusting the pH value in stages to remove iron and aluminum, so as to obtain a goethite slag and an iron-aluminum slag separately; mixing the iron-aluminum slag with an alkali liquor, and heating and stirring same to obtain an aluminum-containing solution and alkaline slag; and heating and stirring the aluminum-containing solution, introducing carbon dioxide thereto and controlling the pH value to obtain aluminum hydroxide and an aluminum-removed solution.
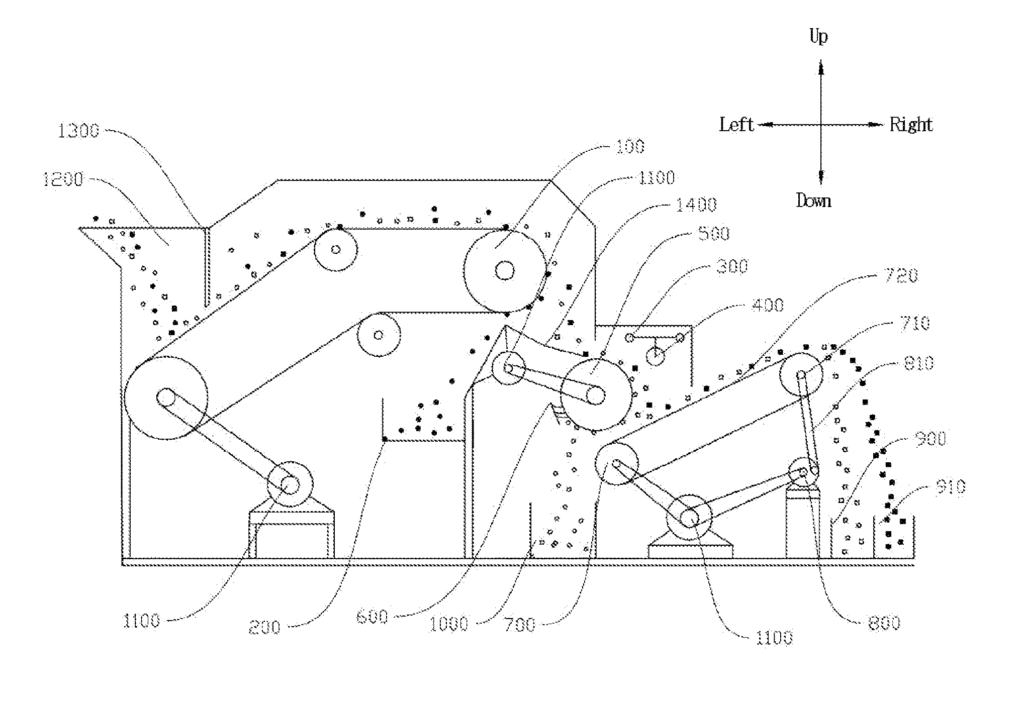
US11759792 — METHOD FOR POWER BATTERY AUTOMATIC FINE-QUANTITY SORTING AND APPARATUS THEREOF — Guangdong Brunp Recycling Technology Co., Ltd., Hunan Brunp Recycling Technology Co., Ltd., and Hunan, Brunp EV Recycling Co., Ltd. (China) — The present invention relates to the technical field of recycling the waste lithium battery, and more particularly, to a method for power battery automatic fine-quantity sorting and an apparatus thereof. It should be understood that the crushed mixture material of the lithium battery has different physical properties. Iron has magnetic properties, and the anode material powder is usually nickel-cobalt-lithium-manganese and lithium-nickel-manganese, having no magnetism or electrical conductivity, while the collector and graphite powder have electrical conductivity. The core of the present invention is to realize physical sorting according to different physical properties of these various materials and discloses a method for power battery automatic fine-quantity sorting and an apparatus thereof, the method including the following steps of S1. The material is crushed, and leveled, and is then subjected to magnetic sorting processing to sort out iron powder; S2. The material after magnetic sorting is subjected to electrostatic processing to sort out positive electrode material powder; S3. The material after electrostatic processing is subjected to bounce processing to sort out the collector and graphite powder. A magnetic sorting device, an electrostatic sorting device, and a bouncing sorting device are accordingly provided.
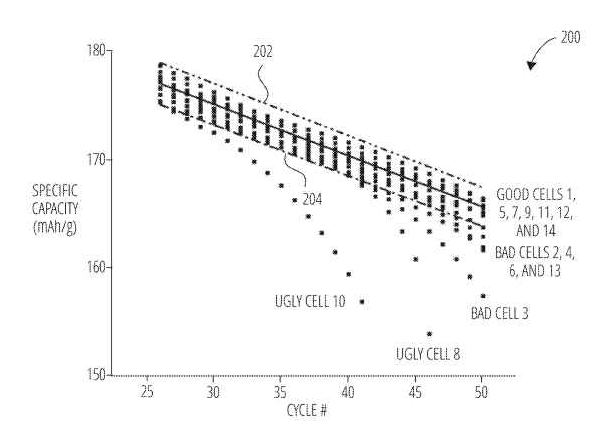
US11740290 — ENERGY STORAGE CELL QUALIFICATION AND RELATED SYSTEMS, METHODS, AND DEVICES — Battelle Energy Alliance, LLC (USA) — Energy storage cell qualification and related systems, methods, and devices are disclosed. A method of qualifying rechargeable battery cells includes taking measurements on the rechargeable battery cells, determining specific capacity distributions of the rechargeable battery cells as a function of a number of discharge cycles based on the measurements, determining one or more specific capacity thresholds to separate the specific capacity distributions of the rechargeable battery cells into two or more classifications, and qualifying the rechargeable battery cells into the two or more classifications based, at least in part, on the specific capacity distributions and the one or more specific capacity thresholds. A method of implementing rechargeable battery cells into product manufacturing and qualifying the rechargeable battery cells and deploying those of the rechargeable battery cells qualified into a first classification of the two or more classifications into the product. Battery-based energy storage systems (BESSs) are some of the most favorable because BESSs may be configured with sufficient flexibility and scale to support multiple purposes and functions and receive the benefits born from the multi-purpose, multi-functionality characteristics. This is particularly true for the lithium-ion battery (LIB)-based BESS (LIBESS).
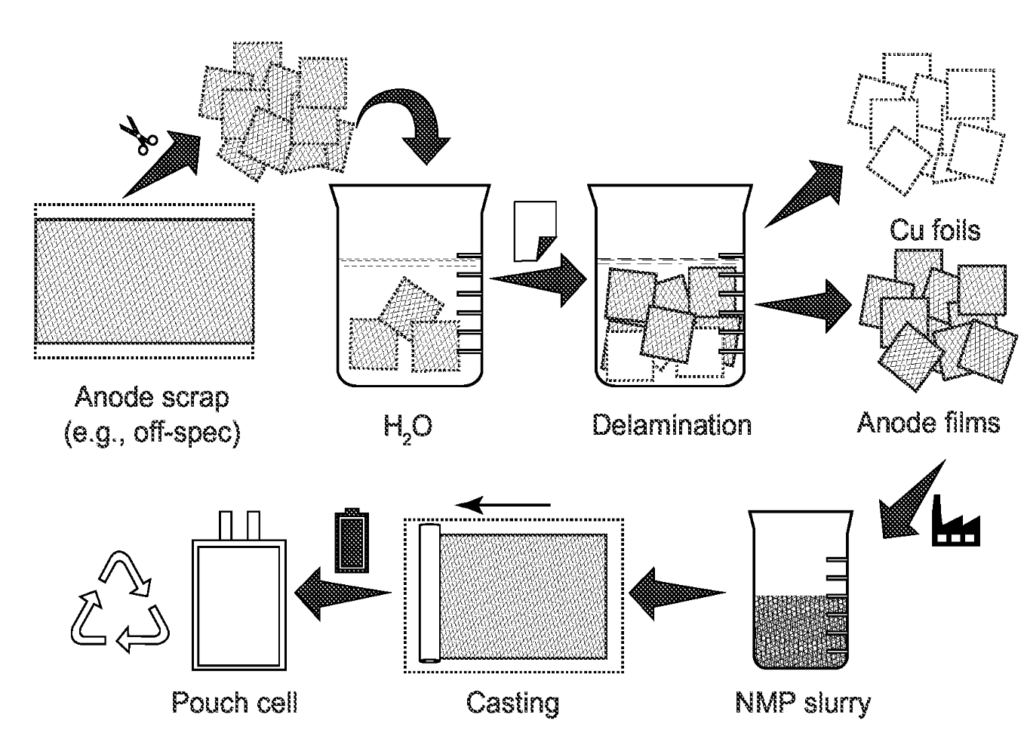
US11721850 — DIRECT RECYCLING OF LITHIUM-ION BATTERY SCRAPS FOR MANUFACTURING A NEW ANODE — UT-Battelle, LLC (USA) — An improved method of recycling lithium-ion battery anode scraps is provided. The method involves isolating an anode scrap including a graphite anode film adhered to a current collector foil with a polyvinylidene fluoride binder. The anode scrap is combined with deionized water to form a first mixture. The graphite anode film is delaminated from the current collector foil to form a second mixture comprising a free collector foil and a free graphite anode film. The free graphite anode film is filtered and dried from the second mixture to recover the free graphite anode film. The free graphite anode film is combined with a solvent comprising N-methyl-2-pyrrolidone (NMP) to form an anode formation slurry. The slurry is coated onto a copper current collector to produce a new anode.
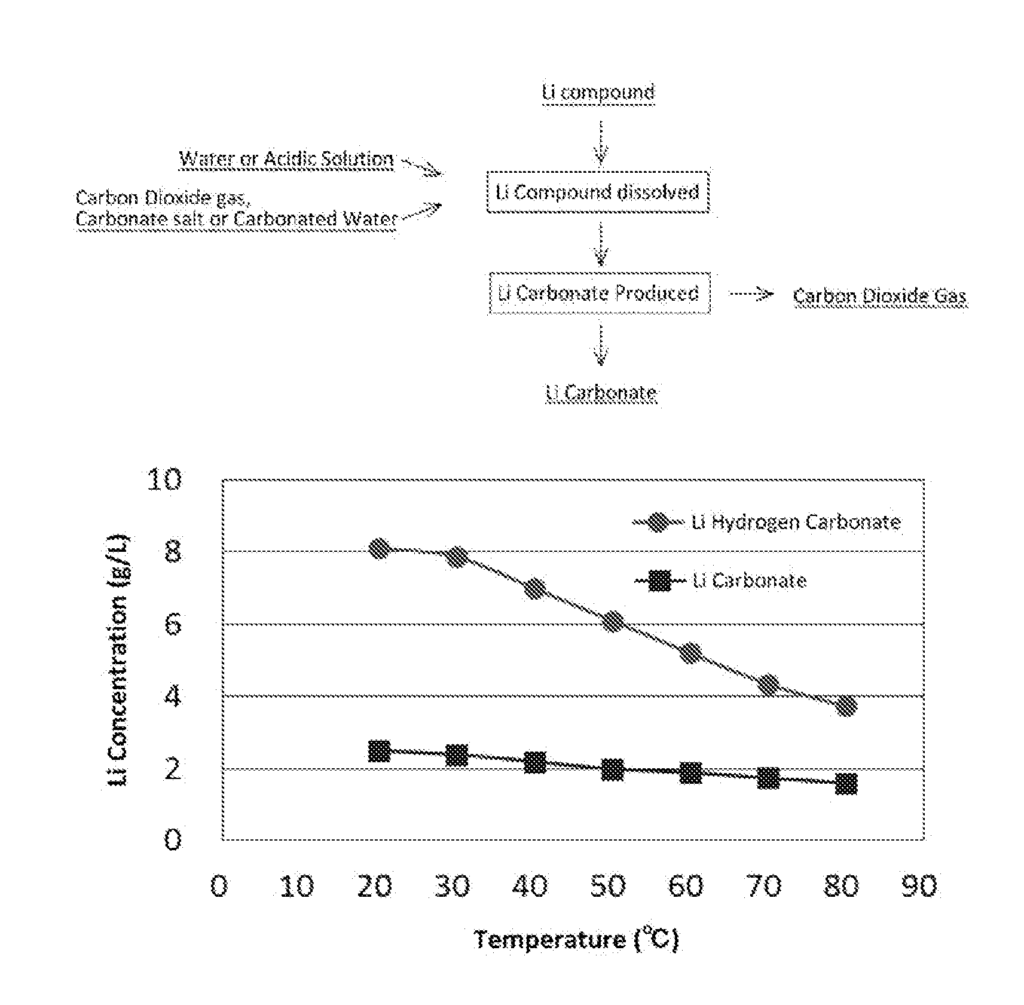
US11718895 — METHOD FOR DISSOLVING LITHIUM COMPOUND, METHOD FOR MANUFACTURING LITHIUM CARBONATE, AND METHOD FOR RECOVERING LITHIUM FROM LITHIUM ION SECONDARY CELL SCRAP — JX Nippon Mining & Metals Corporation (Japan) — A method for dissolving a lithium compound according to the present invention includes bringing a lithium compound into contact with water or an acidic solution, and feeding, separately from the lithium compound, a carbonate ion to the water or the acidic solution to produce carbonic acid, and allowing the carbonic acid to react with the lithium compound to produce lithium hydrogen carbonate. The method for recovering lithium from lithium-ion secondary battery scrap according to the present invention further comprises a lithium precipitating step of separating lithium from a lithium dissolved solution obtained in the lithium dissolving step and precipitating a lithium ion in the lithium dissolved solution as lithium carbonate.
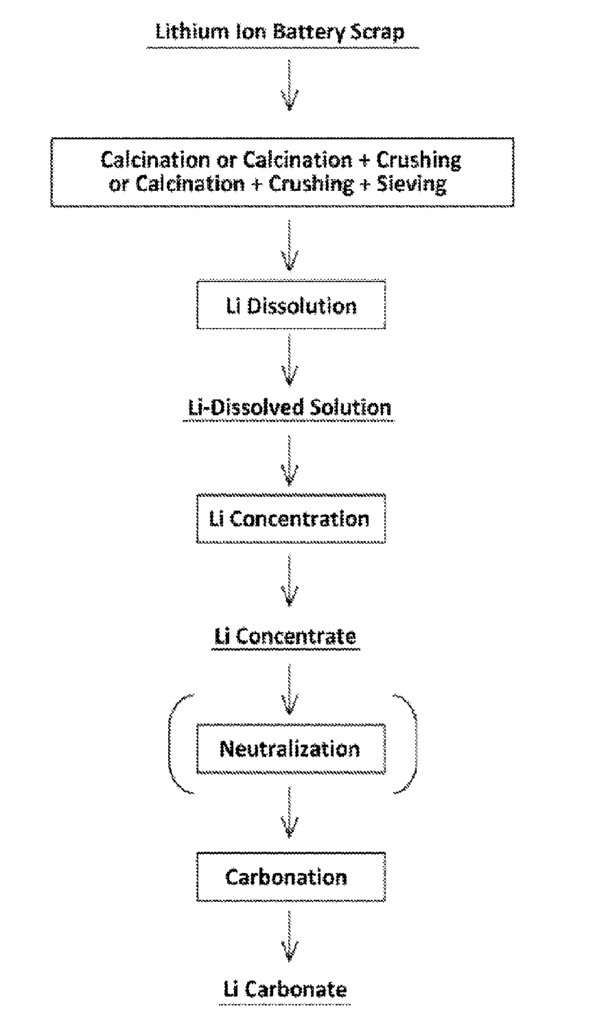
US11702719 — METHOD FOR RECOVERING LITHIUM FROM LITHIUM ION BATTERY SCRAP — JX Nippon Mining & Metals Corporation (Japan) — A method for recovering lithium from lithium ion battery scrap according to this invention comprises subjecting lithium ion battery scrap to a calcination step, a crushing step, and a sieving step sequentially carried out, wherein the method comprises, between the calcination step and the crushing step, between the crushing step and the sieving step, or after the sieving step, a lithium dissolution step of bringing the lithium ion battery scrap into contact with water and dissolving lithium contained in the lithium ion battery scrap in the water to obtain a lithium-dissolved solution; a lithium concentration step of solvent-extracting lithium ions contained in the lithium-dissolved solution and stripping them to concentrate the lithium ions to obtain a lithium concentrate; and a carbonation step of carbonating the lithium ions in the lithium concentrate to obtain lithium carbonate.
US11697885 — PRODUCTION OF NANOPOROUS FILMS — University Of Central Florida Research Foundation, Inc. (USA) — A process is provided comprising submerging a substrate in an electrochemical deposit bath having at least a metal salt and saccharin. In embodiments, the film is further treated with anodization, and in other cases chemical vapor deposition. Films are also provided formed by the disclosed processes. The films are nanoporous on at least a portion of a surface of the films. Also disclosed are electronic devices having the films disclosed, including lithium-ion batteries, storage devices, supercapacitors, electrodes, semiconductors, fuel cells, and/or combinations thereof.
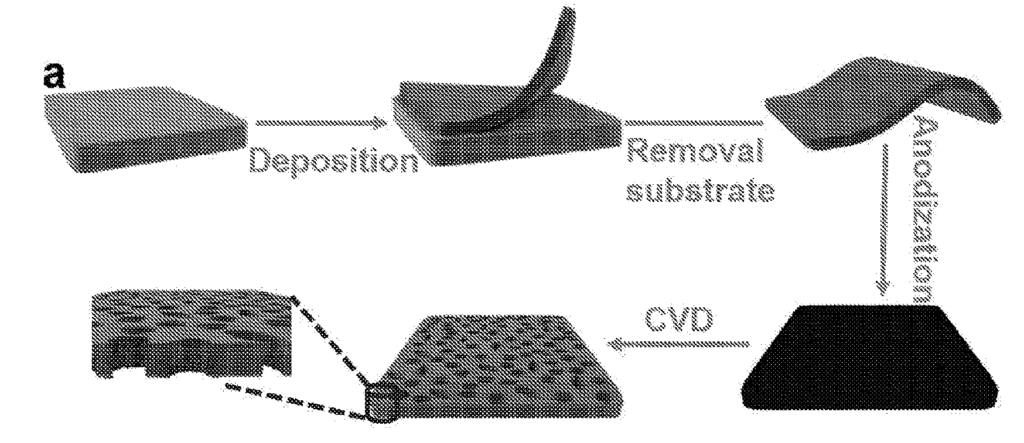
US11695169 — PROCESS FOR THE PREPARATION OF PRECURSOR COMPOUNDS FOR LITHIUM BATTERY CATHODES — Umicore (Belgium) — One of the prevailing lithium-ion battery chemistries involves cathode powders essentially consisting of oxides of the metals lithium, nickel, manganese, and cobalt (NMC). Another heavily used chemistry makes use of cathode powders essentially comprised of lithium, nickel, cobalt and aluminum (NCA), also in the form of oxides. The present disclosure concerns the production of precursor compounds for lithium battery cathodes. Batteries or their scrap are smelted in reducing conditions, thereby forming an alloy suitable for further hydrometallurgical refining, and a slag. The alloy is leached in acidic conditions, producing a Ni- and Co-bearing solution, which is refined. The refining steps are greatly simplified as most elements susceptible to interfere with the refining steps concentrate in the slag. Metals such as Co, Ni and Mn are then precipitated from the solution, forming a suitable starting product for the synthesis of new battery precursor compounds.
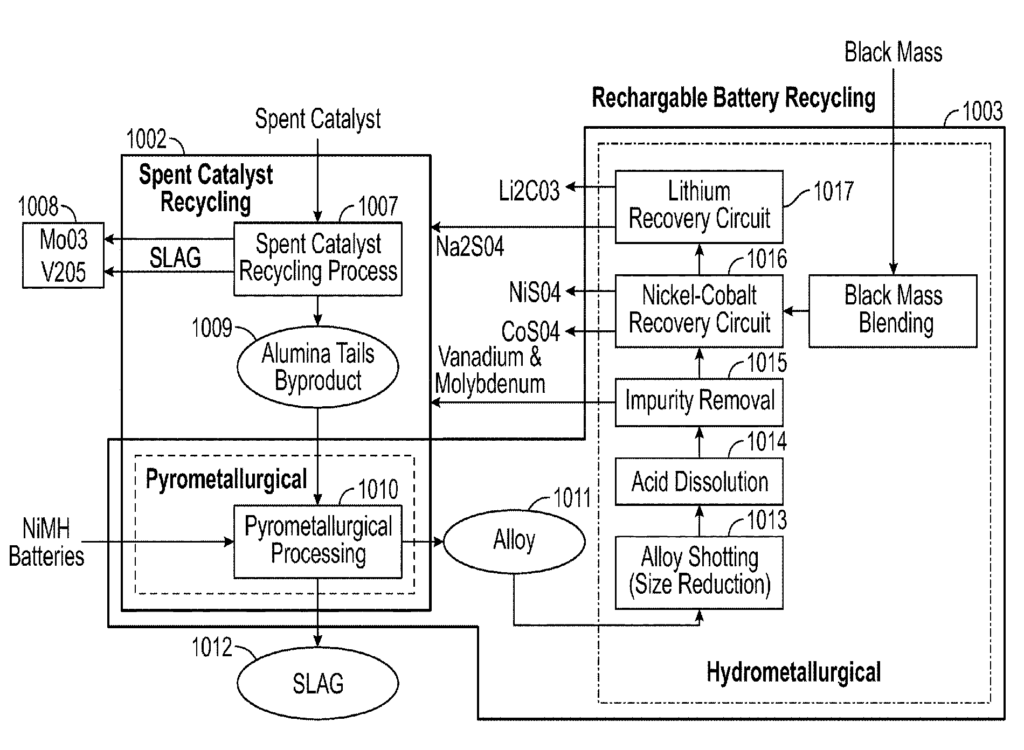
US11682801 — PROCESSES FOR RECYCLING SPENT CATALYSTS, RECYCLING RECHARGEABLE BATTERIES, AND INTEGRATED PROCESSES THEREOF — Aleon Renewable Metals, LLC (USA) — This disclosure is directed to recycling processes, individually and/or integrated, for separating and recovering metals from used hydroprocessing catalyst and for the recycling of rechargeable batteries to yield high purity metals and other commercially viable products and to minimize landfill and other wastes. Integrated recycling method and processes including recycling spent catalyst to produce one or more water-soluble metal salts and one or more water-insoluble tail byproducts, and recycling rechargeable batteries to produce one or more battery-grade metals and one or more pure metallic byproducts, wherein the water insoluble tail byproduct is a feedstock in recycling the rechargeable batteries, the impure metallic byproduct is a feedstock in recycling the spent catalyst, or both.
US11664542 — RECOVERY OF MATERIALS FROM ELECTRODE SCRAPS AND SPENT LITHIUM-ION BATTERIES VIA A GREEN SOLVENT-BASED SEPARATION PROCESS — UT-Battelle, LLC (USA) — A method for recycling lithium-ion battery materials is provided. The method includes isolating a composite electrode comprising an electrode material adhered to a current collector with a polyvinylidene difluoride (PVDF) binder. The composite electrode is combined with triethyl phosphate (TEP) as a solvent to form a mixture. The electrode material is delaminated from the current collector in the mixture to give a free electrode material and a free current collector. Each of the free electrode material and the free current collector is recovered from the mixture. The free electrode material may be reused to prepare another composite electrode, as well as a lithium-ion battery comprising the same, which are also disclosed.
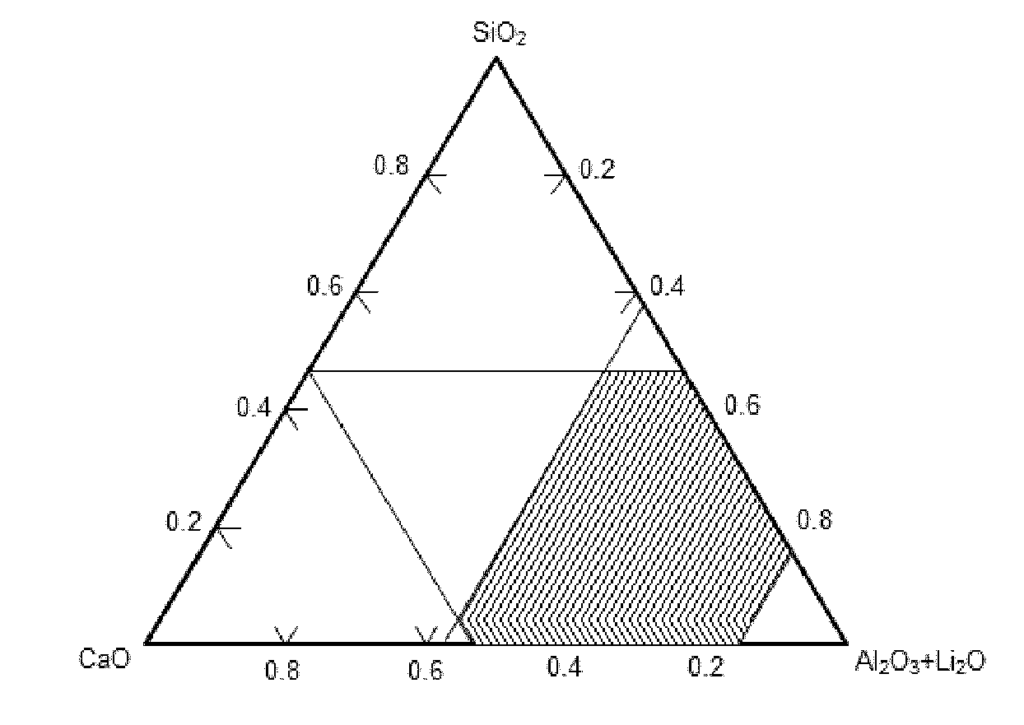
US11661638 — RECOVERY OF NICKEL AND COBALT FROM Li-ION BATTERIES OR THEIR WASTE — Umicore (Belgium) — The present pyrometallurgical invention aims to provide a process for the recovery of Ni and Co from Li-ion batteries, which at the same time increases the lifetime of a furnace, by operating with a dedicated MnO—Li2O-rich slag system composed in a way that magnesia-bearing refractory bricks are less corroded during operation. The present invention lies in the field of pyrometallurgy and discloses a process and a slag suitable for the recovery of Ni and Co from Li-ion batteries or their waste. The slag composition is defined according to:
10%<MnO<40%; (CaO+1.5*Li2O)/Al2O3 >0.3; CaO+0.8*MnO+0.8*Li2O<60%; (CaO+2*Li2O+0.4*MnO)/SiO2≥2.0; Li2≥1%; and, Al2O3 +SiO2+CaO+Li-2O+MnO+FeO+MgO>85%.
This composition is particularly adapted to limit or avoid the corrosion of furnaces lined with magnesia-bearing refractory bricks. The achieved wear reduction contributes nicely to the overall economy of the present process.
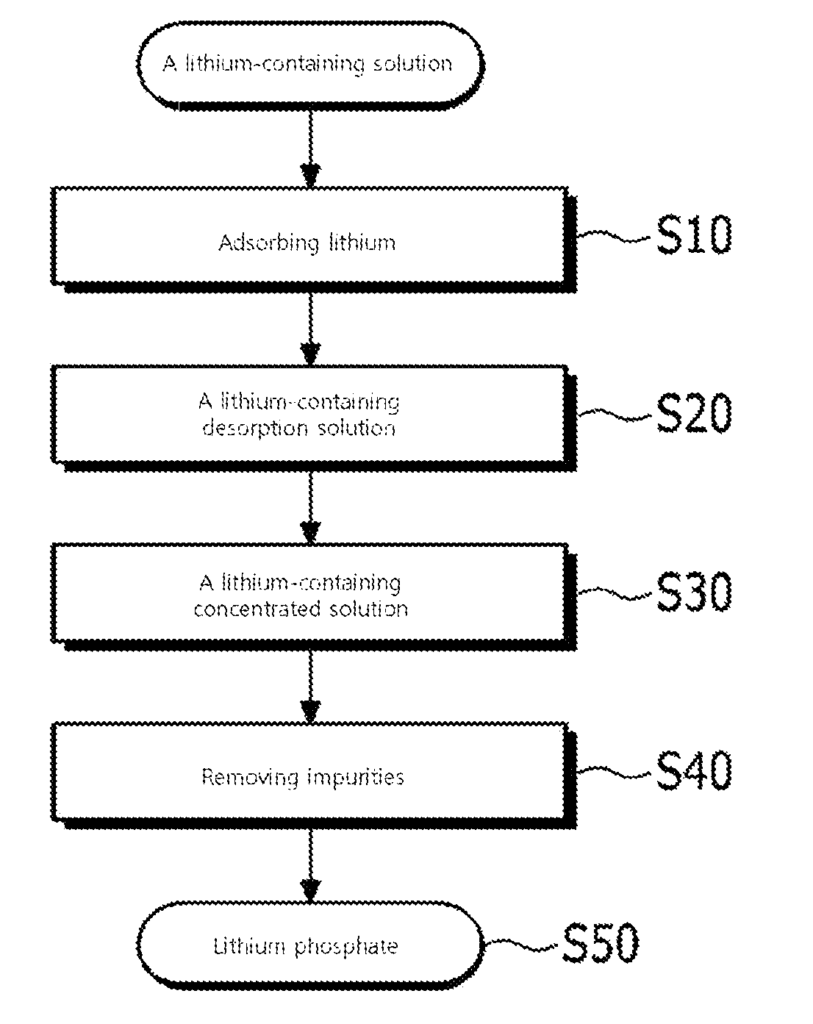
US11655150 — PREPARATION METHOD FOR LITHIUM PHOSPHATE, PREPARATION METHOD FOR LITHIUM HYDROXIDE, AND PREPARATION METHOD FOR LITHIUM CARBONATE — Posco Co., Ltd. and Research Institute of Industrial Science & Technology (Korea) — The present invention relates to a method for producing lithium phosphate, comprising: passing a lithium-containing solution through an aluminum-based adsorbent to adsorb lithium on the aluminum-based adsorbent, passing the distilled water or an aqueous solution having a lower lithium concentration than the lithium-containing solution through the aluminum-based adsorbent on which the lithium is adsorbed to obtain a lithium-containing desorption solution, and putting a phosphorous supplying material in the lithium-containing desorption solution to obtain lithium phosphate, which can be converted to lithium carbonate or lithium hydroxide, core raw materials of secondary lithium batteries.
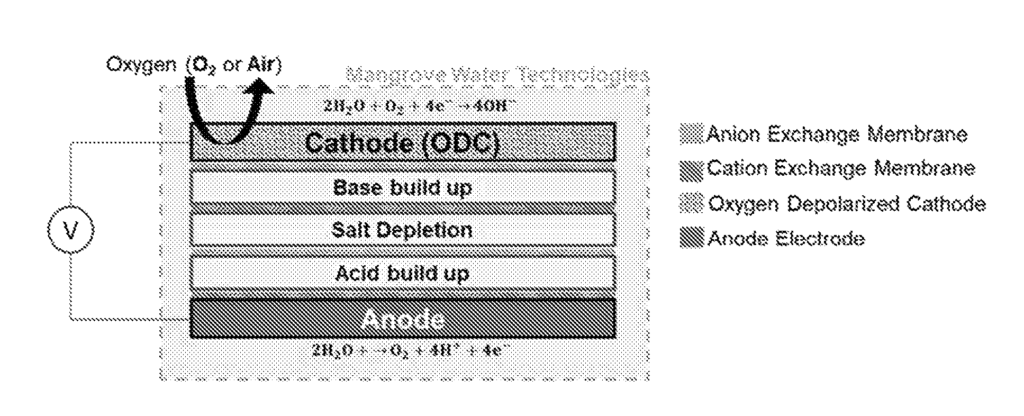
US11634826 — Li RECOVERY PROCESSES AND ONSITE CHEMICAL PRODUCTION FOR LI RECOVERY PROCESSES — Mangrove Water Technologies Ltd. (Canada) — In this disclosure, a process of recycling acid, base and the salt reagents required in the Li recovery process is introduced. A membrane electrolysis cell which incorporates an oxygen depolarized cathode is implemented to generate the required chemicals onsite. The system can utilize a portion of the salar brine or other lithium-containing brine or solid waste to generate hydrochloric or sulfuric acid, sodium hydroxide and carbonate salts. Simultaneous generation of acid and base allows for taking advantage of both chemicals during the conventional Li recovery from brines and mineral rocks. The desalinated water can also be used for the washing steps on the recovery process or returned into the evaporation ponds. The method also can be used for the direct conversion of lithium salts to the high value LiOH product. The method does not produce any solid effluent which makes it easy-to-adopt for use in existing industrial Li recovery plants.
US11605844 — CATHODE RECYCLING OF END-OF-LIFE LITHIUM BATTERIES — Alliance for Sustainable Energy, LLC (USA) — While electric vehicles (EVs) help reduce greenhouse gas emissions, their use also poses several critical concerns. Since the battery cathode is made of some critical and key elements such as cobalt, nickel, manganese, and lithium, shortage of those elements and disruption of the supply chain will be a major concern for mass production. Furthermore, the EV battery packs will generate a significant amount of waste stream after their end-of-life (EOL). Direct cathode recycling tries to address these challenges by recycling and reusing EOL cathode materials. Disclosed herein are improved methods and devices for recycling lithium cathodes from batteries. In an aspect disclosed herein is a method for recycling lithium cathodes from batteries comprising using a redox mediator. A method for recycling lithium cathodes from batteries comprises: providing a battery, removing a lithium containing cathode from said battery, contacting the lithium containing cathode with a redox mediator; wherein the redox mediator is selected from the group consisting of 3,5-di-tert-butyl-o-benzoquinone, thymoquinone, methyl-p-benzoquinone, duroquinone, and naphthoquinone, in order to recover lithium.
US11603579 — LITHIUM-RICH METALLURGICAL SLAG — Umicore (Belgium) — In a known pyrometallurgy process using a molten bath furnace for the recycling of e.g. lithium-bearing batteries, the addition of fluxing compounds leads to the formation of a slag wherein the more easily oxidized elements such as aluminum, silicon and lithium are collected; the less easily oxidized elements such as copper, nickel, and cobalt are collected in a separate alloy phase. The present invention concerns a slag composition having a high lithium content, suitable as additive in the manufacture of end-user products, or for the economic recovery of the contained lithium. The lithium concentration indeed compares favorably with that of spodumene, the classic mineral mined for lithium production. This slag is characterized by a composition according to: 3%<Li2O<20%; 1%<MnO<7%; 38%<Al2O3<65%; CaO<55%; and, SiO2<45%.
US11530133 — METHOD FOR RECOVERING LITHIUM — Industrial Technology Research Institute (Taiwan) — In order to effectively recover lithium ions in seawater, brine, or waste liquid of lithium batteries, the disclosure provides a method for recovering lithium. Through oxidation and reduction reactions, the purpose of high selectivity and rapid absorption and desorption of lithium is achieved. The method includes the following steps. A lithium-containing solution is provided. A manganese oxide adsorbent is immersed in the lithium-containing solution, and a reducing agent is added to carry out an adsorption reaction, and the manganese oxide adsorbent is immersed in a solution containing an oxidizing agent to carry out a desorption reaction.
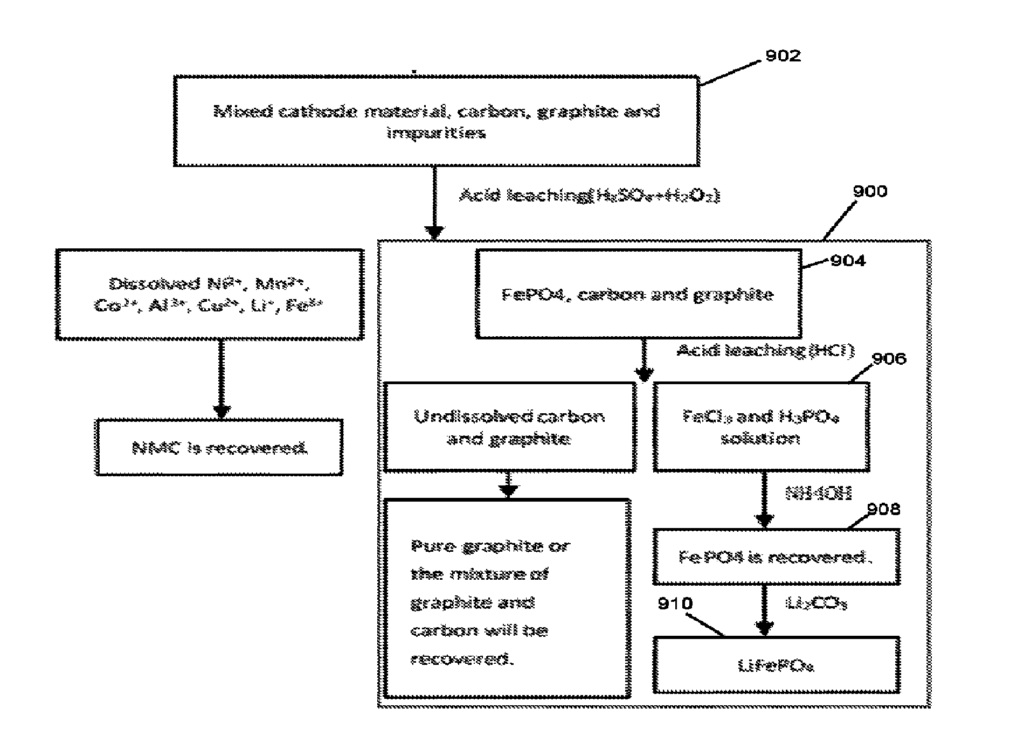
US11509000 — PROCESS FOR THE RECOVERY OF CATHODE MATERIALS IN THE RECYCLING OF BATTERIES BY REMOVING ALUMINUM AND IRON — Northvolt AB (Sweden) — The present disclosure relates to the recycling of batteries, and in particular process steps in the recovery of cathode materials such as lithium (Li), nickel (Ni), manganese (Mn), and cobalt (Co), the latter three frequently referred to as NMC metals. A process for removal of aluminum and iron in the recycling of rechargeable batteries comprising providing a leachate from black mass, adding phosphoric acid (H3PO4) to said leachate and adjusting the pH to form iron phosphate (FePO4) and aluminum phosphate (AlPO4), precipitating and removing the formed FePO4 and AlPO4, and forming a filtrate for further recovery of cathode metals, mainly NMC-metals and lithium.
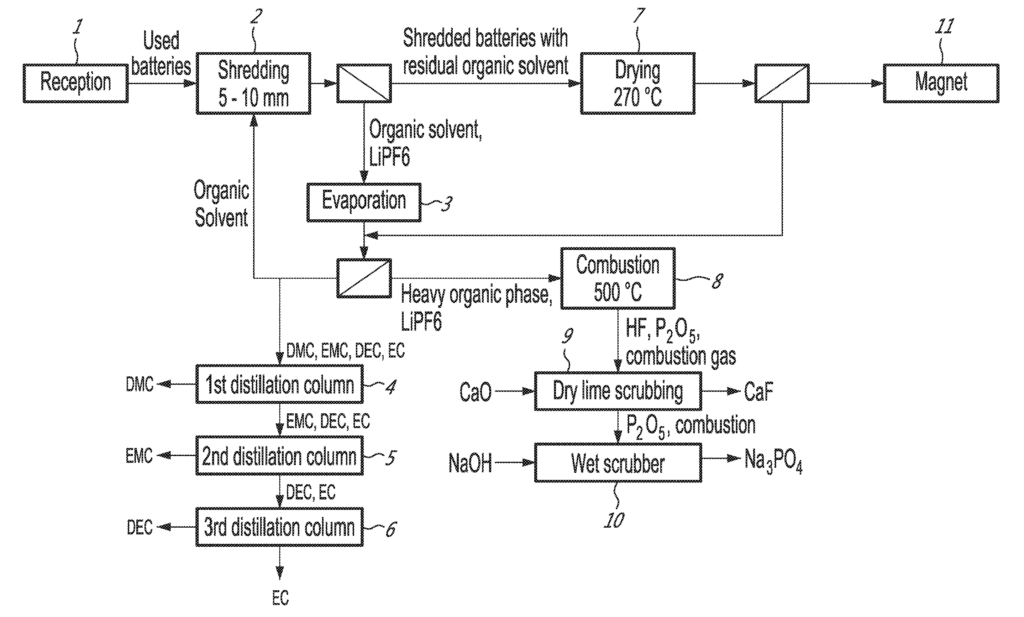
US11508999 — LITHIUM-ION BATTERIES RECYCLING PROCESS — Recyclage Lithion Inc. (Canada) — It is provided a process for recycling lithium ion batteries comprising shredding the lithium-ion batteries and immersing residues in an organic solvent; feeding the shredded batteries residues in a dryer producing a gaseous organic phase and dried batteries residues; feeding the dried batteries residues to a magnetic separator removing magnetic particles; grinding the non-magnetic batteries residues; mixing the fine particles and an acid producing a metal oxides slurry and leaching said metal oxides slurry; filtering the leachate removing the non-leachable metals; feeding the leachate into a sulfide precipitation tank; neutralizing the leachate; mixing the leachate with an organic extraction solvent; separating cobalt and manganese from the leachate using solvent extraction and electrolysis; crystallizing sodium sulfate from the aqueous phase; adding sodium carbonate to the liquor and heating up the sodium carbonate and the liquor producing a precipitate of lithium carbonate; and drying and recuperating the lithium carbonate.
US11502345 — METHOD AND APPARATUS FOR RECYCLING LITHIUM-ION BATTERIES — Worcester Polytechnic Institute (USA) — Cathode material from exhausted lithium ion batteries are dissolved in a solution, including sulfuric acid and a reducing agent, for extracting the useful elements Co (cobalt), Ni (nickel), Al (Aluminum) and Mn (manganese) to produce active cathode materials for new batteries. The solution includes compounds of desirable materials such as cobalt, nickel, aluminum and manganese dissolved as compounds from the exhausted cathode material of spent cells. Depending on a desired ratio, of the desired materials, raw materials are added to the solution to achieve the desired ratio of the commingled compounds for the recycled cathode material for new cells. The desired materials precipitate out of solution without extensive heating or separation of the desired materials into individual compounds or elements. The resulting active cathode material has the predetermined ratio for use in new cells, and avoids high heat typically required to separate the useful elements because the desired materials remain commingled in solution.
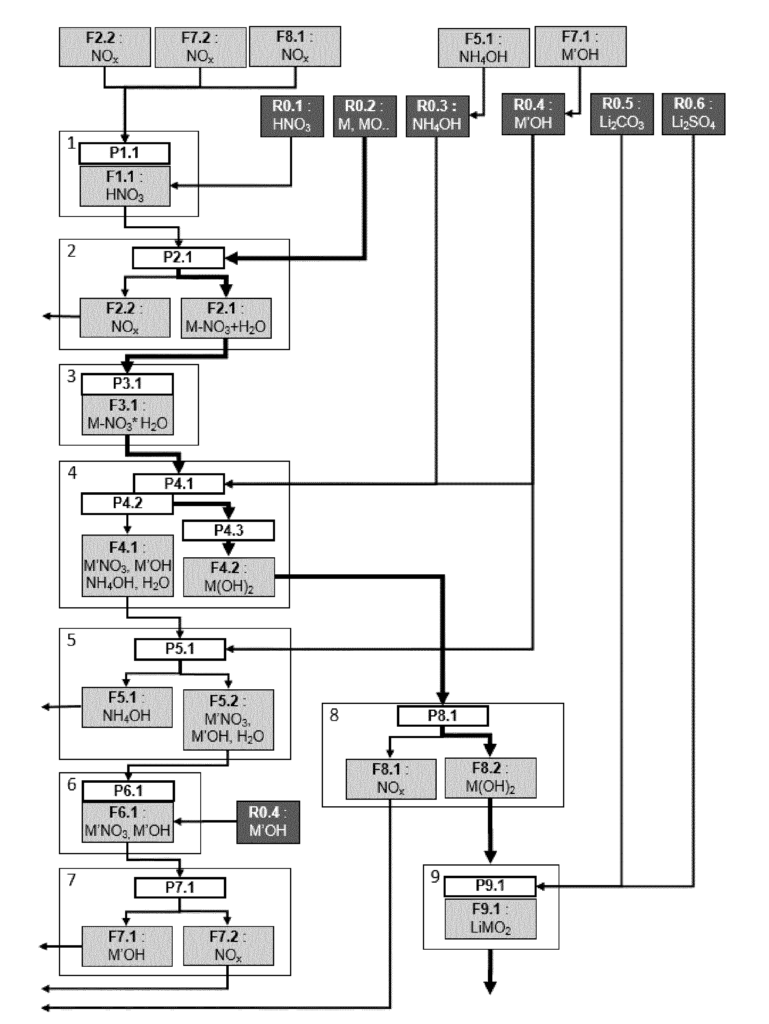
US11401167 — NITRATE PROCESS FOR MANUFACTURING TRANSITION METAL HYDROXIDE PRECURSORS — Umicore (Belgium) and Umicore Korea, Ltd. (Korea) — A strong need to develop processes to produce the cathode materials such as lithium, nickel, manganese, and cobalt oxide layered material, known as “NMC, used in these batteries by a more sustainable process. Generally, due to the low cost of sulfuric acid and of NaOH, there is little financial incentive to recover NaOH from recycled Na2SO4. The generation of waste is therefore a general issue of the sulfate precipitation route. It is especially important to reduce the amount of wastewater, and of its Na2SO4 content. Alternative sustainable co-precipitation processes are strongly needed. This invention relates to an industrial process of manufacturing hydroxide precursor for lithium transition metal oxide used in secondary lithium ion batteries. More particularly, this process utilizes highly concentrated nitrate salts obtained by leaching one or more metals and/or metal compounds using HNO3 in an aqueous solution and is designed to mitigate waste production.
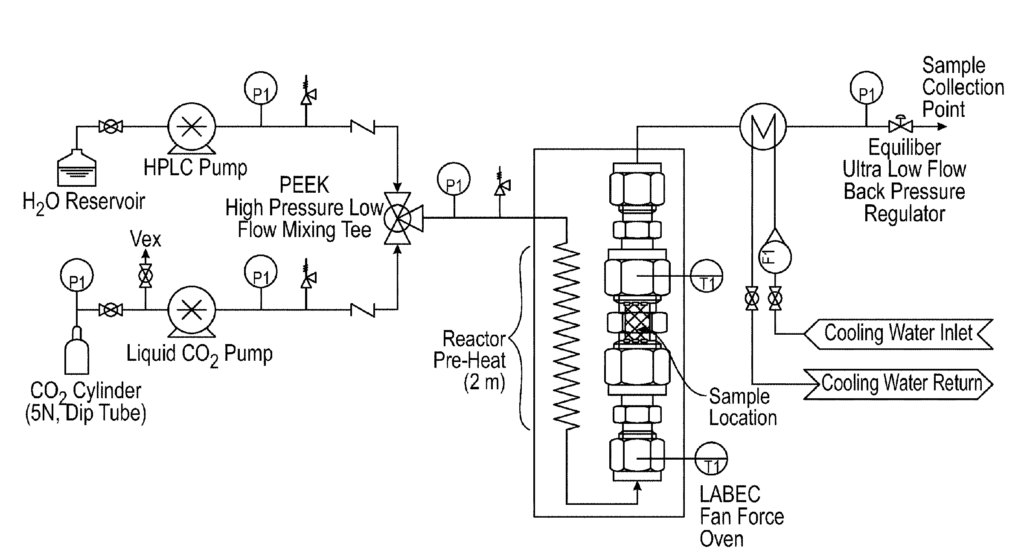
US11371116 — LITHIUM EXTRACTION METHOD — Novalith Technologies Pty Limited (Australia) — According to the present invention there is provided a method for the extraction of lithium from one or more lithium-containing ores such as spodumene, the inventive method comprising the steps of: milling said ore/s to a predetermined average particle size; optionally calcining the milled ore; further optionally performing a secondary milling step; providing an aqueous suspension of the one or more lithium-containing ores at a predetermined solids concentration; subjecting the one or more lithium-containing ores to an aqueous extraction medium defined by a predetermined partial pressure of CO2, a predetermined extraction temperature, over a predetermined time; and obtaining technical grade lithium carbonate/lithium bicarbonate therefrom. Optional concentration and/or precipitation/purification steps may follow.
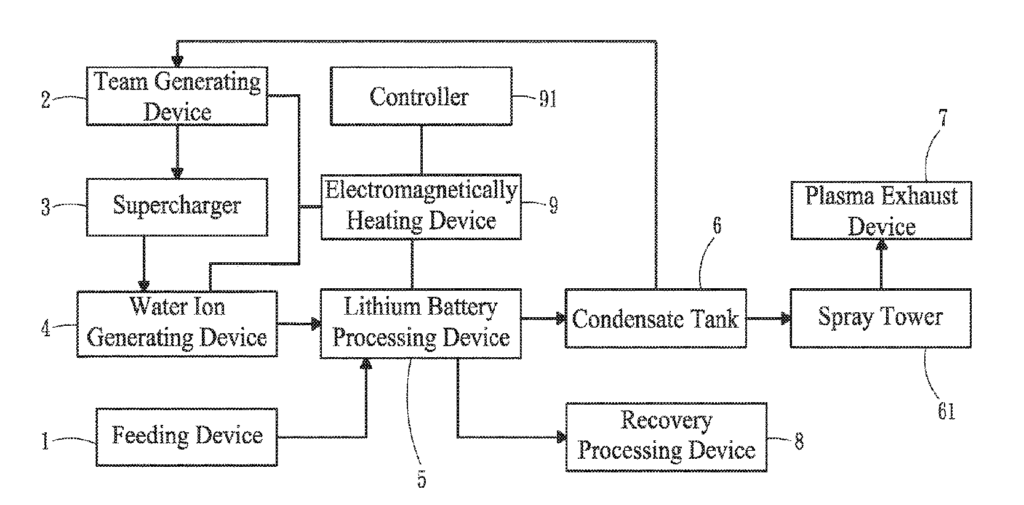
US11316214 — WASTE LITHIUM BATTERY RECOVERY SYSTEM — Yau Fu Industry Co., Ltd. (Taiwan) — The primary objective of the present invention is to provide a waste lithium battery recovery system using pyrolysis of water ions without producing pollution. A waste lithium battery recovery system includes a feeding device, a steam generating device, a supercharger, a water ion generating device, a lithium battery processing device, a condensate tank, a plasma exhaust device, and a recovery processing device. In practice, the steam generating device produces saturated steam. The supercharger heats the saturated steam into superheated steam. The water ion generating device transforms the superheated steam into water ions. The lithium battery processing device performs reactions of molecular scission, pyrolysis and carbonization, and electrolytes and separators of the waste lithium batteries are treated by the water ions to form carbon residues, gas-liquid wastes, and inorganic wastes. The gas-liquid wastes are processed by the condensate tank and the plasma exhaust device to form harmless gases and liquids. The inorganic wastes are processed by the recovery processing device to produce the metals.
US11316157 — METHODS FOR THE PRODUCTION OF CATHODE MATERIALS FOR LITHIUM ION BATTERIES — Ge Solartech, LLC (USA) — The present disclosure provides methods for producing cathode materials for lithium ion batteries. Cathode materials that contain manganese are emphasized. Representative materials include LixNi1-y-zMnyCozO2 (NMC) (where x is in the range from 0.80 to 1.3, y is in the range from 0.01 to 0.5, and z is in the range from 0.01 to 0.5), LixMn2O4 (LM), and LixNi1-yMnyO2 (LMN) (where x is in the range from 0.8 to 1.3 and y is in the range from 0.0 to 0.8). The process includes reactions of carboxylate precursors of nickel, manganese, and/or cobalt and lithiation with a lithium precursor. The carboxylate precursors are made from reactions of pure metals or metal compounds with carboxylic acids. The manganese precursor contains bivalent manganese and the process controls the oxidation state of manganese to avoid formation of higher oxidation states of manganese.
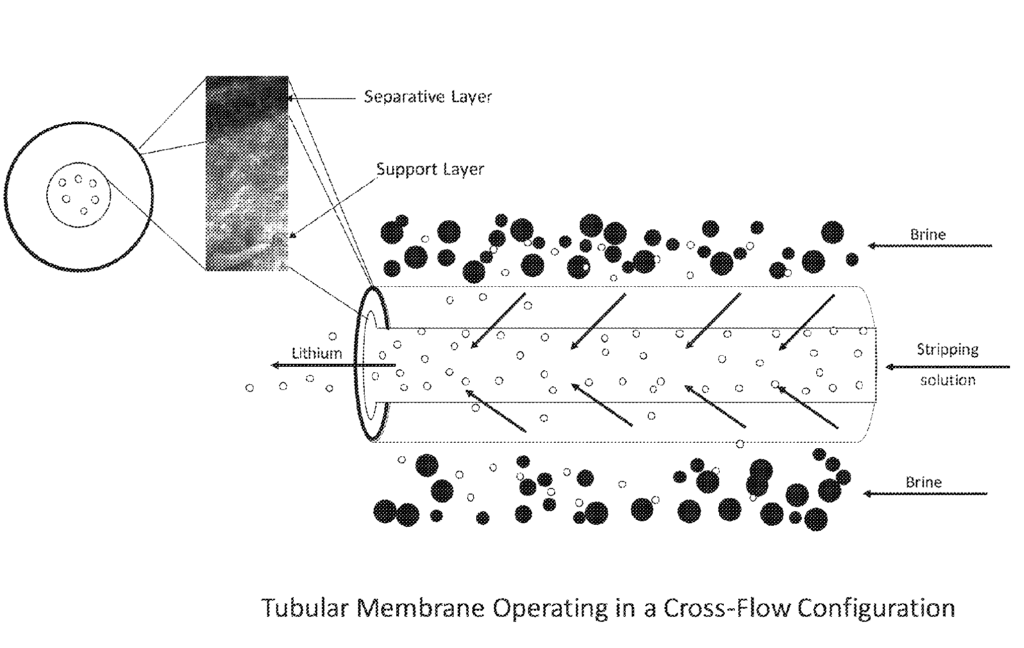
US11253820 — LITHIUM EXTRACTION COMPOSITE FOR RECOVERY OF LITHIUM FROM BRINES, AND PROCESS OF USING SAID COMPOSITION — UT-Battelle, LLC (USA) and All American Lithium LLC (USA) — A lithium extraction composite comprising: (i) a porous support and (ii) particles of a lithium-selective sorbent material coated on at least one surface of the support, wherein the support has a planar membrane, fiber (or rod), or tubular shape. A method for extracting and recovering a lithium salt from an aqueous solution by use of the above-described composition is also described, the method comprising (a) flowing the aqueous source solution through a first zone or over a first surface of the lithium extraction composite to result in selective lithium intercalation in the lithium-selective sorbent material in the first zone or first surface; and (b) simultaneously recovering lithium salt extracted in step (a) from said lithium-selective sorbent material by flowing an aqueous stripping solution through a second zone or over a second surface of the lithium extraction composite in which lithium ions from the first zone or first surface diffuse.
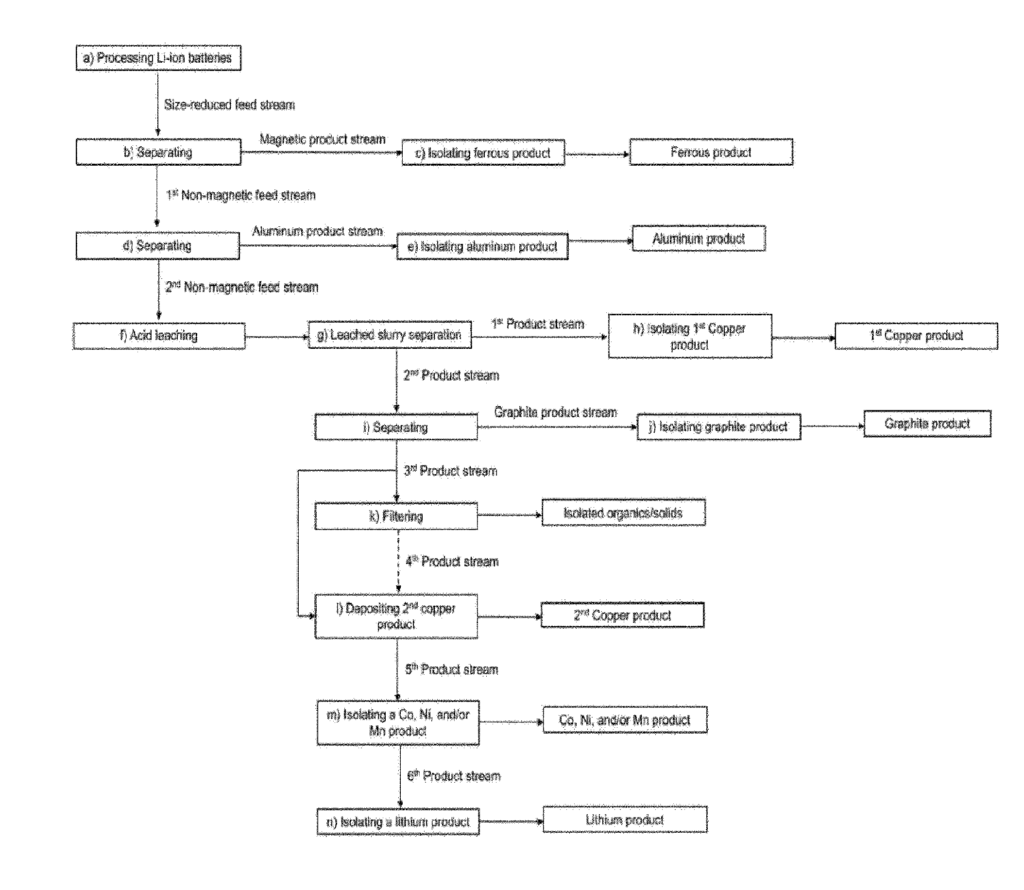
US11135595 — PROCESS, APPARATUS, AND SYSTEM FOR RECOVERING MATERIALS FROM BATTERIES — Li-Cycle Corp. (Canada) — The present application pertains to the field of battery recycling. More particularly, the present application relates to a process, apparatus, and system for recovering materials from batteries, in particular rechargeable lithium-ion batteries. An apparatus for carrying out size reduction of battery materials under immersion conditions can include a housing configured to hold an immersion liquid comprising at least one of sodium hydroxide and calcium hydroxide. A first feed chute may define an opening therein for receiving battery materials of a first type into the housing and a first submergible comminuting device may be disposed within the housing and submerged in the immersion liquid to receive the battery materials of the first type from the first feed chute. The first submergible comminuting device may be configured to cause a si ze reduction of the battery materials of the first type to form a first reduced-size battery material.
US11050098 — PROCESS FOR THE RECYCLING OF LITHIUM BATTERY ELECTRODE MATERIALS — Hydro-Quebec (Canada) — A process for the recycling of an electrochemically active material from used lithium metal and lithium-ion batteries is described. The process comprises the steps of reacting the electrochemically active material with an oxidizing agent or a reducing agent in a solvent without addition of a strong acid to produce a lithium salt and a delithiated electrochemically active material precipitate. This precipitate is separated from the lithium salt and used in the regeneration of the electrochemically active material. The process comprises the steps of: (a) reacting the electrochemically active material with an oxidizing agent or a reducing agent in a solvent (e.g. an aqueous solvent), to produce a lithium salt and an electrochemically active material precursor precipitate; (b) separating the electrochemically active material precursor precipitate from the lithium salt; and (c) regenerating the electrochemically active material from the electrochemically active material precursor. In one embodiment, the solvent is water.
US10919046 — PROCESS, APPARATUS, AND SYSTEM FOR RECOVERING MATERIALS FROM BATTERIES — Li-Cycle Corp. (USA) — An apparatus for carrying out size reduction of battery materials under immersion conditions having a battery inlet and at least a first comminuting device disposed within a housing and configured to cause a size reduction of the battery materials to form reduced-size battery materials and to liberate electrolyte materials and a black mass material comprising anode and cathode powders from within the battery materials. An immersion liquid can be within the housing and can submerge the first comminuting device so the black mass material and the reduced-size battery material are entrained within the immersion liquid to form a sized-reduced feed stream. A feed outlet may be downstream from the first comminuting device.
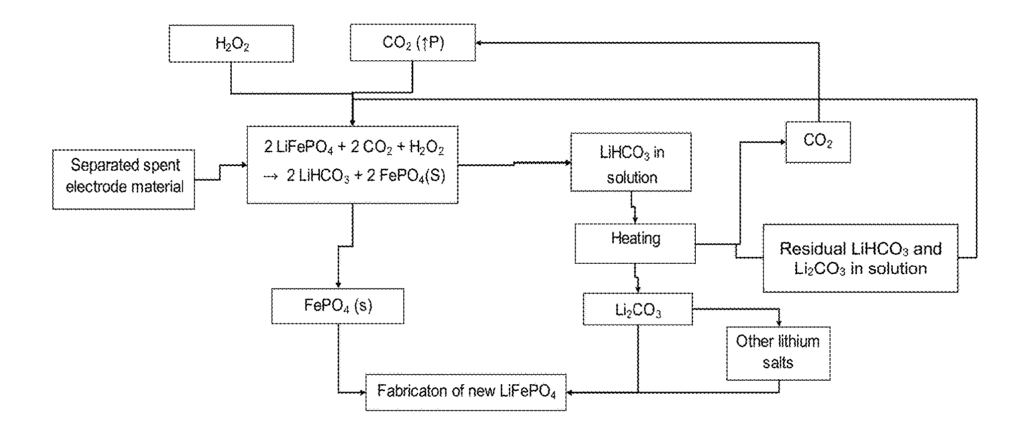
US10741890 — METHOD AND APPARATUS FOR RECYCLING LITHIUM IRON PHOSPHATE BATTERIES — Worcester Polytechnic Institute (USA) — Cathode material from exhausted lithium-ion batteries is dissolved in a solution for extracting the useful elements Co (cobalt), Ni (nickel), Al (Aluminum) and Mn (manganese) to produce active cathode materials for new batteries. The solution includes compounds of valuable charge materials such as cobalt, nickel, aluminum and manganese dissolved as compounds from the exhausted cathode material of spent cells. However, LiFePO4 is a waste stream charge material often discarded due to infeasibility of recycling. LiFePO4 is precipitated as FePO4 and remains as a by-product, along with graphite and carbon, which are not dissolved into the solution. FePO4 can be separated from graphite and carbon, FePO4 can be used to synthesize LiFePO4 as cathode materials and graphite can be regenerated as anode materials.
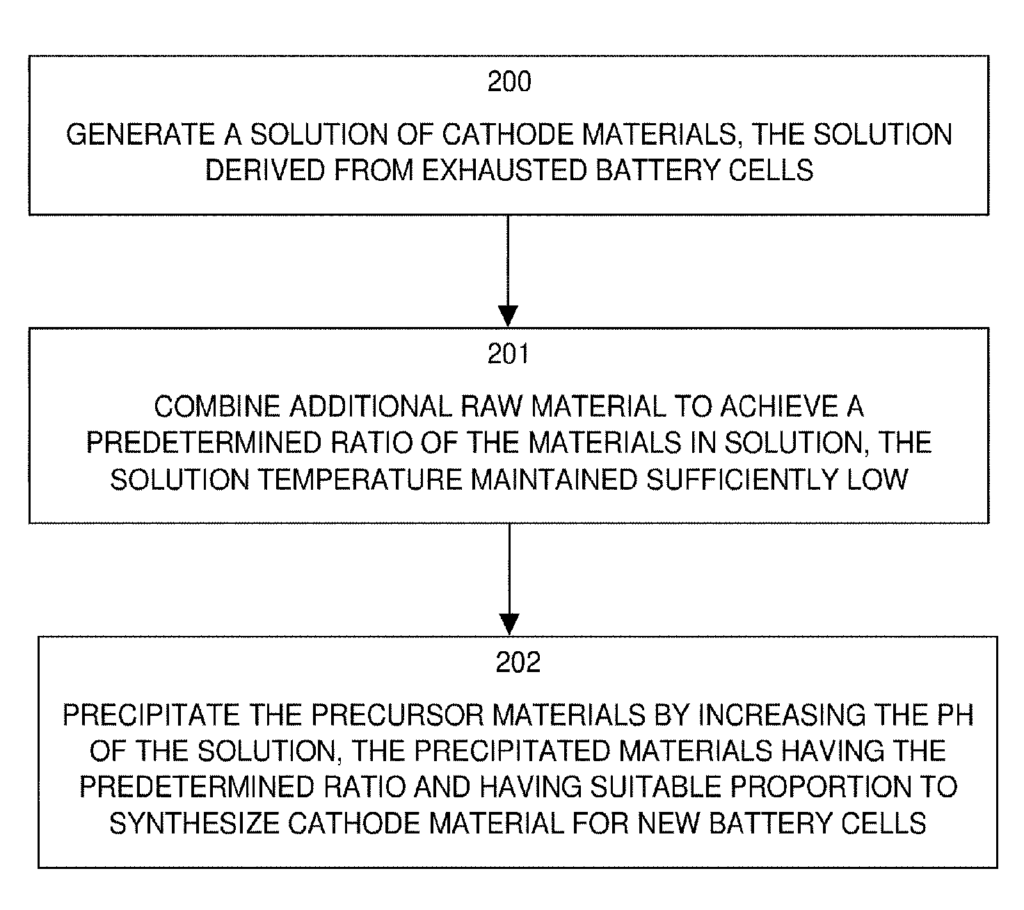
US10522884 — METHOD AND APPARATUS FOR RECYCLING LITHIUM-ION BATTERIES — Worcester Polytechnic Institute (USA) — A method of recycling Li-ion batteries includes generating a solution of aggregate battery materials from spent cells, and precipitating mixtures from the generated solution. Cathode material from exhausted lithium-ion batteries is dissolved in a solution for extracting the useful elements Co (cobalt), Ni (nickel), Al (Aluminum) and Mn (manganese) to produce active cathode materials for new batteries. The solution includes compounds of desirable materials such as cobalt, nickel, aluminum and manganese dissolved as compounds from the exhausted cathode material of spent cells. Depending on a desired ratio of the desired materials, raw materials are added to the solution to achieve the desired ratio of the commingled compounds for the recycled cathode material for new cells. The desired materials precipitate out of solution without extensive heating or separation of the desired materials into individual compounds or elements. The resulting active cathode material has the predetermined ratio for use in new cells, and avoids high heat typically required to separate the useful elements because the desired materials remain commingled in solution.
US10490866 — METHOD FOR ACID DISSOLUTION OF LiCoO2 CONTAINED IN SPENT LITHIUM-ION BATTERIES — Consejo Nacional De Investigaciones Científicas Y Técnicas (Conicet) (Argentina), Universidad Nacional De Cuyo (Argentina), and Inis Biotech LLC (USA) — A method for the acid dissolution of LiCoO2 contained in the cathode of lithium ion batteries, using acetic or tartaric acid as leaching agent, the method being characterized in that it comprises a first stage and a second stage, wherein said first stage comprises the step of separating the cathode components, while said second stage comprises the step of dissolving the pure LiCoO2 with at least one acid. The method allows achieving an economically viable complete recycling process with low environmental impact.