Editors Note: This survey focuses on primary aluminum production patents granted recently in the U.S. and Canada, neighboring countries working together since the industry began. Today, Canada is one of the largest aluminum exporters to the U.S. A large majority of Canada’s primary aluminum is exported to the U.S., making it an important raw material for the country’s manufacturing industries. Both countries have witnessed tremendous global changes and developments in the primary aluminum industry in the 21st century and remain important trading partners across the entire value chain. This is especially significant relative to primary aluminum production.
As described in a recent U.S. Congressional report on aluminum manufacturing: “Through the year 2000, the United States ranked as the world’s largest producer of primary aluminum. In 2021, the United States accounted for less than 2% of global primary aluminum production and ranked as the ninth-largest primary aluminum producing country.” This decline was inevitable, as primary aluminum smelting is highly dependent on electricity availability (some 40% of its production cost), with the result that the U.S. is a high-cost primary aluminum producer. This lack of primary aluminum smelting means that the U.S. is basically dependent on imports.1
Compared to the U.S., Canada has relatively low energy/electricity costs and, by using mostly hydroelectricity and the latest generation technology, Canadian primary aluminum producers have the lowest carbon footprint in the world compared to that of the U.S. and other large producers.2 The nine primary aluminum plants in the Quebec province and the one in Kitimat, British Columbia, produced some 3.0 million tonnes of primary aluminum in 2022, making it the fourth largest global primary aluminum producer. Canadian exports of aluminum products were valued at $18.2 billion in 2022 ($12.1 billion was unwrought alloyed and non-alloyed aluminum) with the U.S. being Canada’s largest export destination for aluminum products in 2022, accounting for 92% of the total value of aluminum exports.3
The primary U.S. and Canadian aluminum patents selected here were granted just over the last year and supplement the primary aluminum patent reviews published in the February 2020, 2022, and 2023 LMA issues. As was mentioned then, the Elysis partnership, a 50:50 joint venture between Alcoa and Rio Tinto, has been developing a technology to produce primary aluminum via the Hall-Héroult process using inert anodes that produce oxygen rather than carbon dioxide from carbon anodes during electrolysis, along with other innovative technologies. The Elysis R&D center is located at the site of the Arvida R&D center in Saguenay, Quebec, Canada, and has already made much inventive progress. Production ready, the inert anode technology will no doubt make the primary aluminum industry sustainable not only in North America, but also internationally.
References
1. Watson, C.D., U.S. Aluminum Manufacturing: Industry Trends and Sustainability, Congressional Research Service Report R47294 (Prepared for Members and Committees of Congress), Oct. 26, 2022, https://crsreports.congress.gov.
2. Hasanbeigi, A., et al., “Aluminum Climate Impact: An International Benchmarking of Energy and CO2 Intensities,” Global Efficiency Intelligence Report, February 2022.
3. “Aluminum Facts,” Government of Canada.
— Joseph C. Benedyk, Editor
US11811229 — METHOD AND SYSTEM FOR CONTROLLING AN ELECTRICAL INSTALLATION — ES ⋅ FOR ⋅ IN SE (Germany) — A method for controlling an electrical installation at least one of an electrical energy source or an energy sink connected to a power source. Industrial consumers such as aluminum smelters can have the energy switched off (load shedding) and switched on again by remote control to stabilize the grid frequency and/or the grid voltage. The electrical installation is coupled to a power grid. The method includes a period of time having a start time and a duration being specified, an upward flexibility Fo, which includes a forecast maximum feed-in power increase or feed-out power decrease, and a downward flexibility Fu, which includes a forecast maximum feed-out power increase or feed-in power decrease, being set for the period of time, a selling threshold price Pv and a purchasing threshold price Pe being set for the period of time, and an electricity trading transaction being concluded for the period of time. The electricity trading transaction includes a base value, base quantity, base price, and date on which the electricity trading transaction is to be carried out.
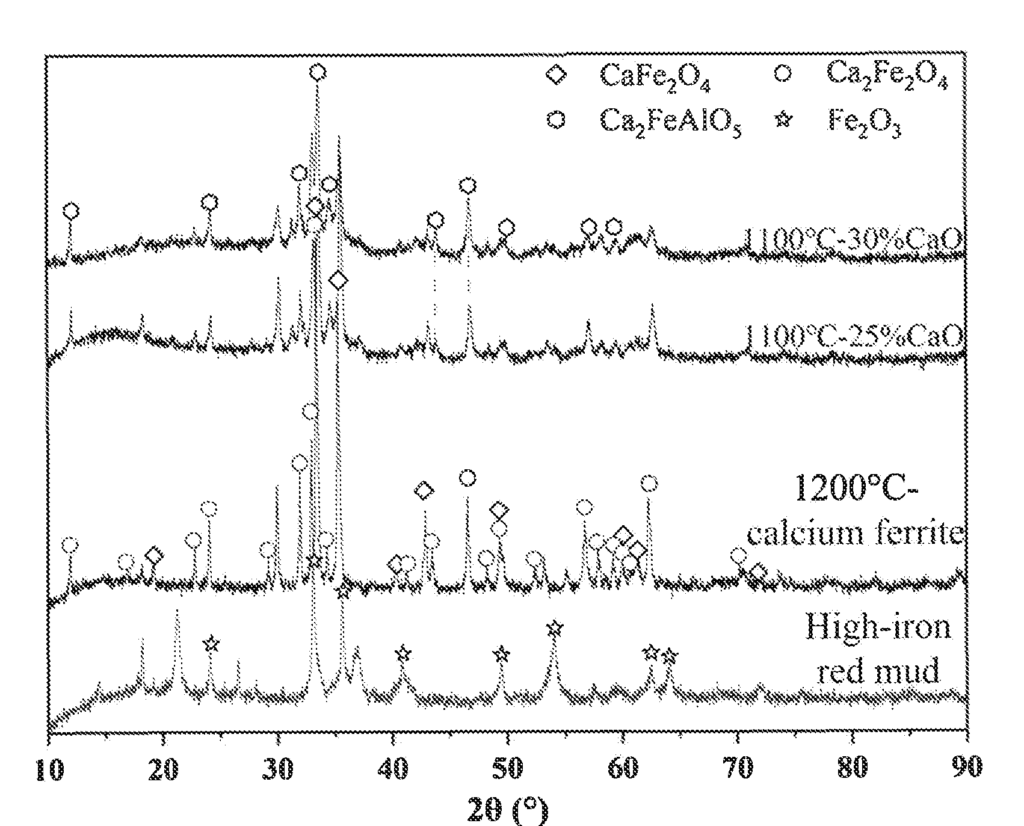
US11773025 — RED MUD-BASED COMPOSITE CALCIUM FERRITE AND PREPARATION METHOD AND USE THEREOF — University of Science and Technology Beijing (China) — Provided is a red mud-based composite calcium ferrite and a preparation method and use thereof. The preparation method of the red mud-based composite calcium ferrite includes the following steps: mixing red mud and a calcium source, and roasting an obtained mixture in an oxygen-containing atmosphere to obtain the red mud-based composite calcium ferrite; where the calcium source is selected from the group consisting of lime and calcium carbonate. In the present disclosure, the composite calcium ferrite is prepared using a solid waste red mud produced by aluminum production, with a greatly reduced cost of raw materials; on the other hand, compared with traditional calcium ferrite, the composite calcium ferrite mainly has phase structures of CaFe2O4, Ca2FeAlO5, and Ca2Fe2O5. Therefore, the composite calcium ferrite has a lower melting point, a higher lime dissolution efficiency, and better fluxing and dephosphorization effects during primary smelting and refining of molten steel.
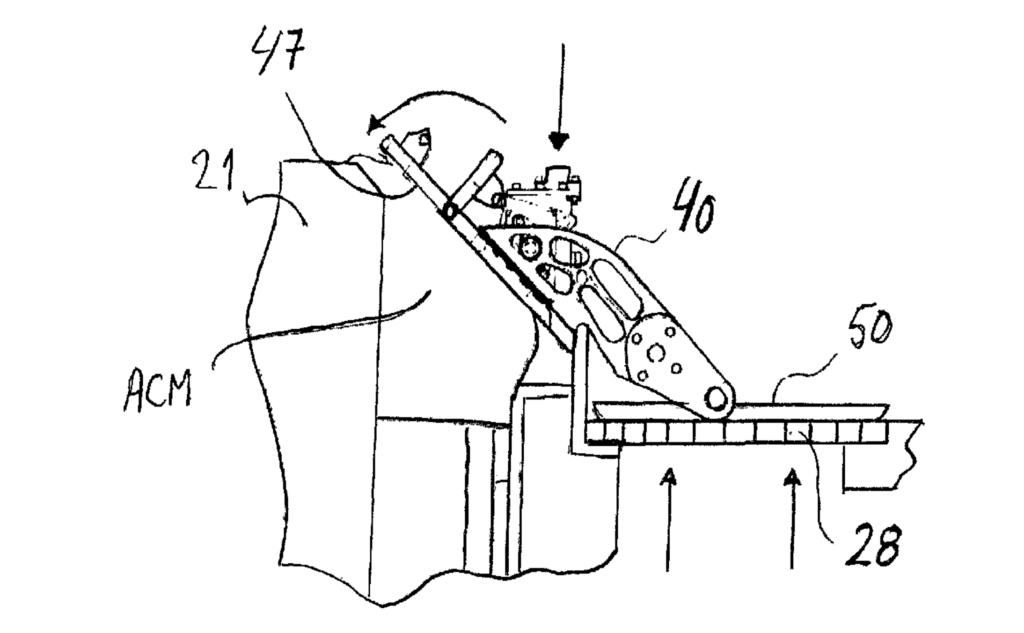
US11746432 — METHOD AND MEANS FOR APPLICATION OF ANODE COVERING MATERIAL (ACM) IN AN ELECTROLYSIS CELL OF HALL-HEROULT TYPE FOR ALUMINUM PRODUCTION — Norsk Hydro ASA (Norway) — A method and means for application of anode covering material (ACM) in an electrolysis cell for aluminum production where the cell being of Hall-Héroult type with prebaked anodes. The cell contains a cathode pot with a rectangular footprint and a superstructure with a gas collecting hood that lays onto the top of the cathode pot. A floor construction at least substantially surrounds the cell at a level below the top of the cathode pot and ventilation openings provided with grates are arranged in the floor in the close vicinity to the cell. The superstructure’s hood is provided with removable lids that are removed for giving access to the cell’s anodes through openings. ACM is applied via a feed tube to cover the anodes and the deposit of ACM is supported by a shuttering. The purpose is to protect the anodes from unwanted oxidation and air-burn, to thermally insulate the electrolyte from heat losses into the superstructure above it, and the layer of ACM will also contribute to control of fluoride losses.
US11746431 — METAL INERT ANODE FOR ALUMINUM PRODUCTION OF BY THE ELECTROLYSIS OF A MELT — Obshchestvo s Ogranichennoy Otvetstvennosty yu “Obedinennaya Kompaniya Rusal Inzhenerno-Tekhnologicheskiy Tsentr” (Russia) — The design of a metal inert anode is proposed, it is made in the form of a perforated structure with through-openings, in particular formed by longitudinal and transverse anode elements intersecting each other and limited by the lateral sides of the intersecting anode elements, and contains vertical or inclined fins that protrude from the bath and are integrated with the anode elements or a current conductor. As a result, it ensures a reduction in the voltage drop in the anode and in the bubble layer under the anode, a reduction in the anode overvoltage and anode consumption, an increase in current efficiency and the reliability of the cryolite-alumina crust, which leads to an increase in the anode service life and promotes the formation of a reliable and durable cryolite-alumina crust above the melt surface, which improves process efficiency.
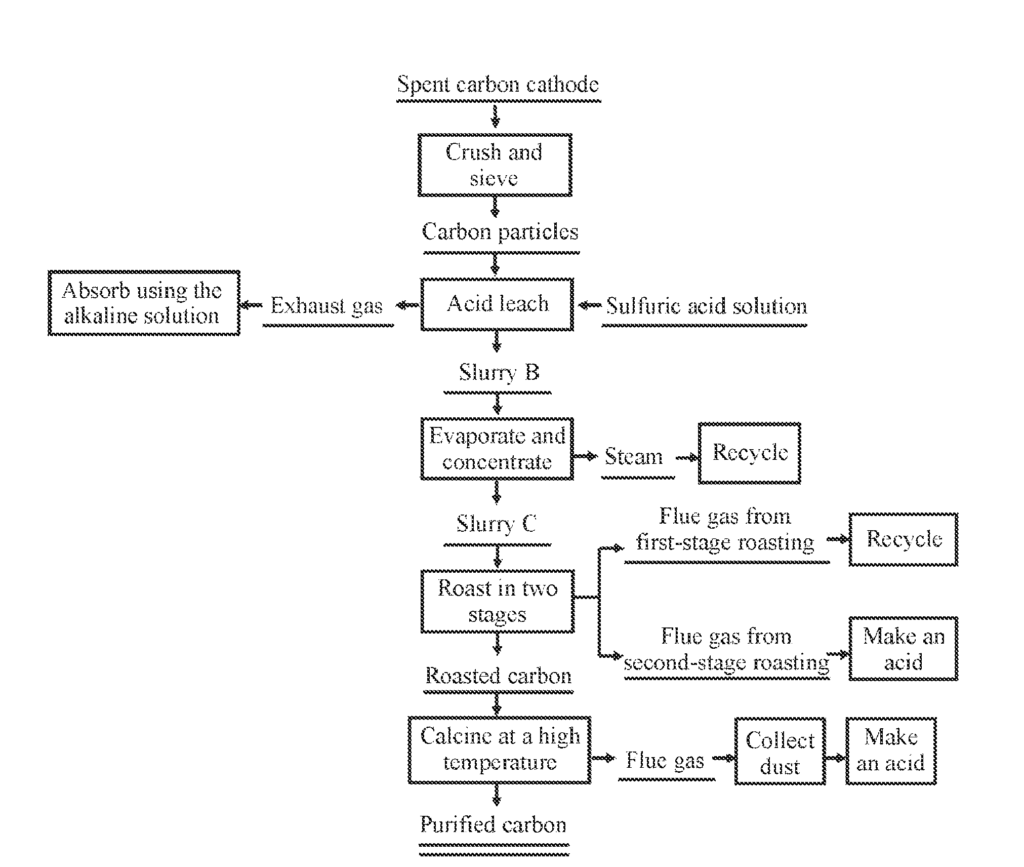
US11697594 — METHOD FOR RECYCLING SPENT CARBON CATHODE OF ALUMINUM ELECTROLYSIS — Central South University (China) — A method for recycling spent carbon cathode of aluminum electrolysis includes the following steps: (1) crushing and sieving spent carbon cathode, to obtain carbon particles; (2) mixing the carbon particles with a sulfuric acid solution, to obtain a slurry A, and then performing pressure leaching, to obtain a slurry B; (3) evaporating and concentrating the slurry B until a mass percentage of water is lower than 8%, to obtain a slurry C; (4) adding concentrated sulfuric acid to the slurry C to obtain a slurry D, then roasting the slurry D at 150-300°C for 0.5-10 h, and then roasting at 300-600°C for 0.5-8 h, to obtain the roasted carbon; and calcining the roasted carbon at a high temperature, to obtain the purified carbon, or mixing the roasted carbon with a leaching agent, and performing leaching, filtering, and washing, to obtain the purified carbon.
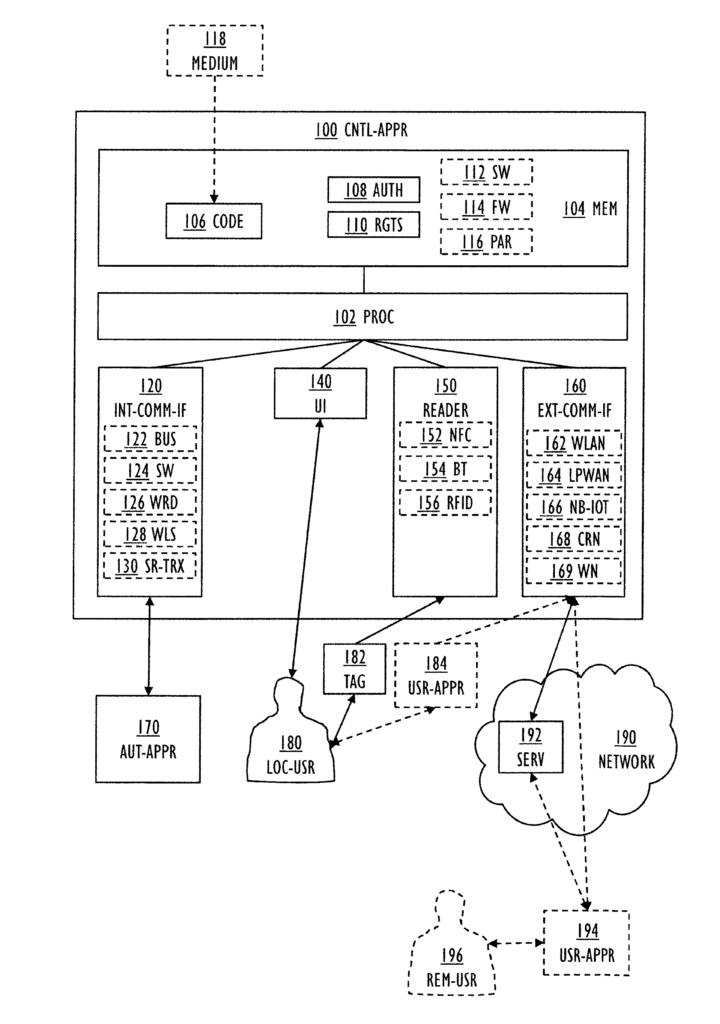
US11693942 — ACCESS CONTROL APPARATUS AND METHOD FOR CONTROLLING CONFIGURATION OF AUTOMATION APPARATUS — ABB Schweiz AG (Switzerland) — An access control apparatus and method for controlling a configuration of an automation apparatus. The method includes: reading authentication information from an electronic tag; transmitting the authentication information to a networked service; receiving access rights from the networked service; and controlling the configuration of the automation apparatus according to the access rights. The automation apparatus 170 may be used in process control and automation solutions of numerous industries including, but not limited to aluminum production.
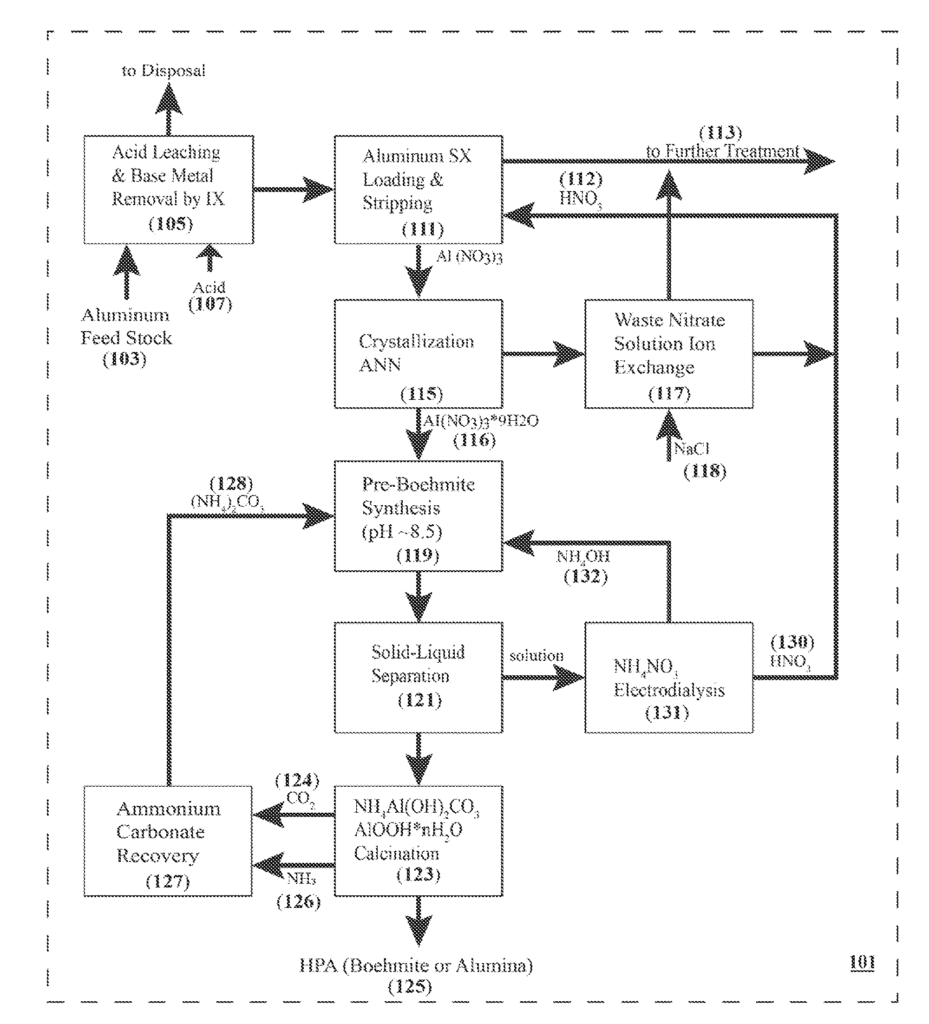
US11691888 — PROCESS FOR THE PREPARATION OF HIGH PURITY ALUMINA — Scandium International Mining Corporation (USA) — A method for preparing high purity alumina (HPA) is provided. The method includes subjecting an aluminum feedstock to acid leaching, thereby yielding an aluminum bearing leachate; subjecting the aluminum bearing leachate to solvent extraction, thereby yielding an organic phase which is loaded with aluminum; stripping the aluminum from the loaded organic phase with a stripping solution containing an acid, thereby yielding an aluminum bearing extract; crystallizing an aluminum salt from the aluminum bearing extract; dissolving the aluminum salt in an ammoniacal solution, thereby generating a boehmite precursor compound and an ammonium salt; calcining the boehmite precursor compound to yield HPA; subjecting the ammonium salt to electro-dialysis, thereby yielding ammonia and the acid; and performing at least one step of (a) utilizing the ammonia in preparing the ammoniacal solution used in a subsequent iteration of the method, or (b) utilizing the acid in preparing the stripping solution used in a subsequent iteration of the method.
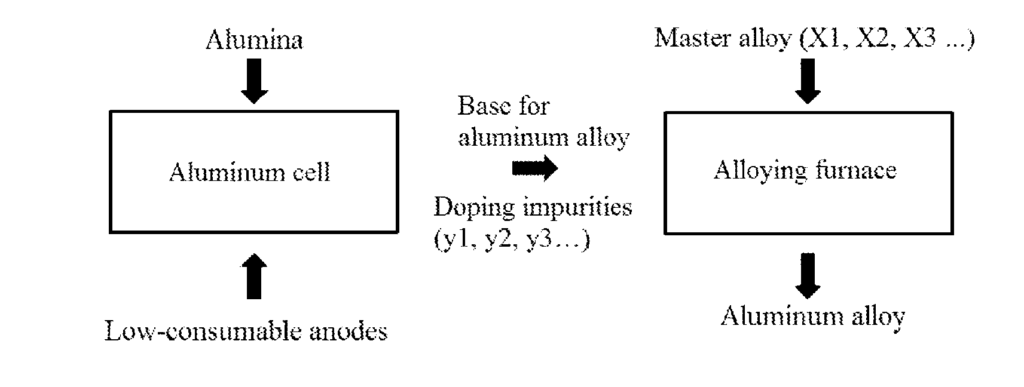
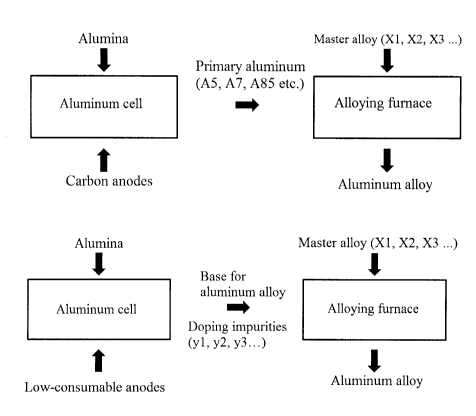
US11634829 — METHOD FOR PRODUCING ALUMINUM ALLOYS — Obshchestvo s Ogranichennoy Otvetstvennosty yu “Obedinennaya Kompaniya Rusal Inzhenerno-Tekhnologicheskiy Tsentr” (Russia) — The invention relates to production of alloys based on aluminum. A method is proposed for producing aluminum-based alloys by electrolysis, according to which low-consumable anode of aluminum pot is used as a source of alloying elements. At the same time, in order to optimize master alloy consumption, one of the following options is chosen: dissolution of alloying elements from slightly soluble anodes; adding oxides and/or fluorides and/or carbonates of alloying elements to electrolyte melt of aluminum pot; simultaneous dissolution of alloying elements from slightly soluble anodes with addition of oxides and/or fluorides and/or carbonates of alloying elements to electrolyte melt of aluminum pot. The method comprises the following stages: introducing alloying elements into molten cathode aluminum by dissolving them in electrolyte melt of aluminum pot from low-consumable anode and/or by adding oxides/and fluorides and/or carbonates of alloying elements into electrolyte melt of aluminum pot; reduction of alloying elements introduced into electrolyte melt of aluminum pot on molten cathode aluminum to form the base for aluminum alloys; determining percentage of elements in the base for aluminum alloys; and bringing alloys to a given composition by adding alloying elements to the base for aluminum alloys in the required amount. The result is multicomponent aluminum alloys of a given composition with introduction of alloying admixtures in the process of aluminum production by electrolysis, and then the alloy is brought to a predetermined composition, providing simplification of technology and control, reducing master alloy consumption which leads to lower cost of aluminum alloy production.
US11630081 — METHOD FOR NON-DESTRUCTIVELY EXAMINING AN ANODE OF AN ALUMINUM ELECTROLYSIS CELL — Obshchestvo s Ogranichennoy Otvetstvennosty yu “Obedinennaya Kompaniya Rusal Inzhenerno-Tekhnologicheskiy Tsentr” (Russia) — The method for non-destructive flaw detection of aluminum reduction cell anodes is claimed, including the building of a computed model of an anode or the use of a specified model with the known data on the geometry and specific resistance of the anode, the geometry and coordinates of internal defects, wherein several cyclic calculations are carried out; the results of calculations are represented in the form of a 3D-matrix of amplitudes and directions of vectors of calculated intensities or inductions of the electromagnetic field at the discretization points near the outer surface of the anode; at least a pair of electrically conductive contacts that supply the specified amount of electrical current through the anode are placed on the outer surfaces of the inspected anode; at least one sensor is placed near the outer surface of the inspected anode, and the amplitude and direction of the magnetic field intensity or induction vectors are measured and represented as a 3D-matrix of measured magnetic field intensity or induction vectors; the 3D-matrices of calculated and measured magnetic field intensity or induction vectors at the same discretization points near the outer surface of the anode are compared; and, based on results, the sizes and coordinates of internal defects are observed. As a result, the informational value and accuracy of determining the location of defects are increased; the process capabilities of the method are expanded by reducing the instability of transition resistances of the contact area in the stub holes of the anode; the confidence and reliability of flaw detection by measuring the magnetic field intensity vectors with contactless sensors are improved.
US11629419 — ANODE FOR ALUMINUM ELECTROLYSIS — R + D Carbon Ltd. (Switzerland) — It is the object of the invention to create an anode for aluminum electrolysis that provides for a reduced voltage drop in particular in an early stage of the anode life. Further, it is an object of the invention to create an anode, wherewith an optimized current density distribution can be achieved. An anode, in particular an anode for the use in aluminum electrolysis cells, includes an anode body with a first stub hole for the insertion of a stub for the connection with a voltage source. The anode includes at least a first aluminum core and a second aluminum core that are arranged inside the anode body for the connection with the voltage source. A first distance between the first aluminum core and the bottom of the anode is different from a second distance between the second aluminum core and the bottom of the anode.
US11566336 — METHOD FOR TRANSFORMING A CRYSTAL FORM OF AN ELECTROLYTE CONTAINING LITHIUM FOR ALUMINUM ELECTROLYSIS — Northeastern University (China) — A method for transforming a crystal form of an electrolyte containing lithium for aluminum electrolysis includes the following steps: S1, pulverizing the electrolyte containing lithium; S2, uniformly mixing an additive with the electrolyte powder to obtain a mixture, wherein the additive is one or more selected from the group consisting of an oxide of an alkali metal other than lithium, an oxo acid salt of an alkali metal other than lithium, and a halide of an alkali metal other than lithium; a molar ratio of a sum of alkali metal fluoride contained in the electrolyte, alkali metal fluoride directly added from the additive, and alkali metal fluoride to which the additive is converted under the high-temperature calcination condition in the mixture to aluminum fluoride is greater than 3; S3, calcining the mixture at a high temperature.
US11566335 — FORMATION OF LINING LAYERS IN THE CATHODE SHELLS OF ALUMINUM ELECTROLYTIC REDUCTION CELLS — Obshchestvo s Ogranichennoy Otvetstvennosty yu “Obedinennaya Kompaniya Rusal Inzhenerno-Tekhnologicheskiy Tsentr” (Russia) — The invention relates to non-ferrous metallurgy and the electrolytic production of aluminum and can be used for lining the cathode assembly of an electrolytic cell. The present method consists in laying materials while simultaneously distributing same over the surface of a base and levelling them at a height measured from the plane of the top edge of the shell of the cathode assembly of the electrolytic cell by gradually moving a device for installing unformed lining materials along the longitudinal axis of the cathode of the aluminum electrolytic cell. Said device is configured in the form of a bridge equipped with a mechanical drive for movement. The bridge has guides on which a frame is mounted for vertical movement, said frame having cassettes provided with gates with a mechanical drive. The technical result is reduced labor costs, healthier working conditions for operatives, and a quality installation of the base of an electrolytic cell.
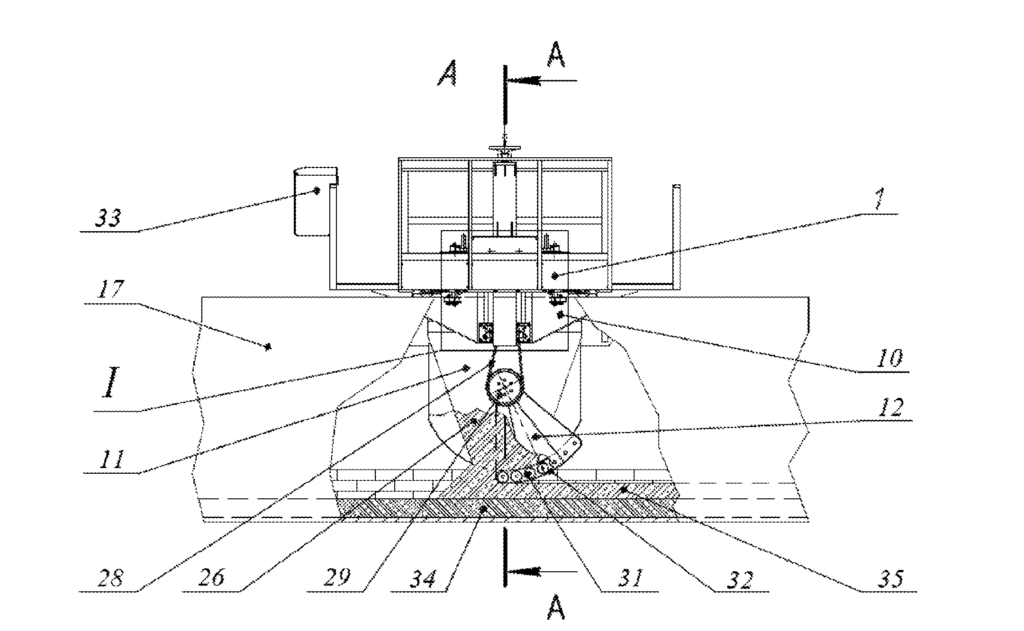
CA3148080 — ALUMINUM REDUCTION CELL WITH A HEAT INSULATED SIDE LINING — Obshchestvo S Ogranichennoy Otvetstvennost’yu “Obedinennaya Kompaniya Rusal Inzhenerno-Tekhnologicheskiy Tsentr” (Russia) — The invention relates to non-ferrous metallurgy, and more particularly to a design for electrolytic cells for the electrolytic production of aluminum and can be implemented in all types of electrolytic cells. The present electrolytic cell comprises a metallic cathode shell, a heat-insulating lining and a refractory lining, a bottom made of bottom blocks with conductive cathode posts, and a side wall lining made of silicon carbide plates with an additional cast refractory layer, the latter having lower heat conductivity and being disposed between the walls of the metallic cathode shell and the silicon carbide plates. The result is: a reduction in the operating voltage of the electrolytic cell by virtue of a reduction in heat loss from the side walls thereof; stabilization of the heat balance; and an increase in the MHD stability of the electrolytic cell.
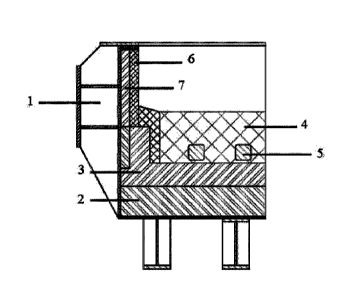
CA3143359 — APPARATUS AND METHOD FOR OPERATING AN ELECTROLYTIC CELL — Elysis Limited Partnership (Canada) — An apparatus, also named transfer box or TB, for conveying an anode assembly outside of an electrolyte cell is described. An apparatus, also named cell preheater lifting beam or CPLB, for conveying an anode assembly or a cell pre-heater outside of an electrolyte cell is also disclosed. TB and CPLB are conjointly used for starting up the electrolytic cell or for replacing a spent anode assembly while maintaining the production of non-ferrous metal, such as aluminum or aluminum. The thermal insulation of the TB allows maintaining the anode temperature homogeneity and preventing thermal shocks when introducing the inert anodes into the hot electrolytic bath. TN and CPLB allow accurate positioning of anode assemblies or cell-preheaters over the electrolysis cell before achieving mechanical and electrical connections of the anode assembly or the cell pre-heater to the electrolysis cell. Several related methods for the operation of an electrolytic cell are also disclosed.
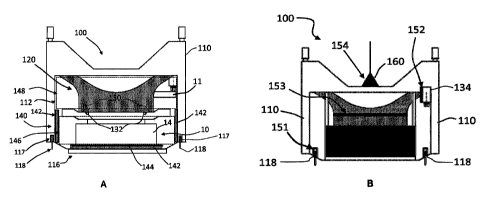
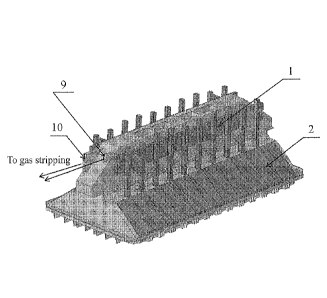
CA3132212 — SYSTEM AND METHOD FOR COLLECTING AND PRE-TREATING PROCESS GASES GENERATED BY AN ELECTROLYSIS CELL — Elysis Limited Partnership (Canada) — Apparatus and method for collecting and pretreating process gases produced in an electrolytic cell during aluminum production are disclosed. The apparatus comprises a collecting unit configured to draw off primary process gases from the electrolytic cell, for instance by drawing-off primary process gases from orifices purposely made over the electrolytic bath; and a pre-treating unit fluidly connected to the collecting unit and configured to receive a fluid bed of fluorinated alumina for pre-treating the primary process gases. The collecting and pre-treating units are within or immediately aside the electrolytic cell in the potroom. The apparatus can be combined with a gas treatment center (GTC) located outside the potroom. Among other advantages, the technology allows collecting primary process gases directly at electrolytic bath level, separating primary process gases and hood-space process gases to pretreat the primary process gases with alumina before the GTC, and using fluid bed reactors without filter bags.
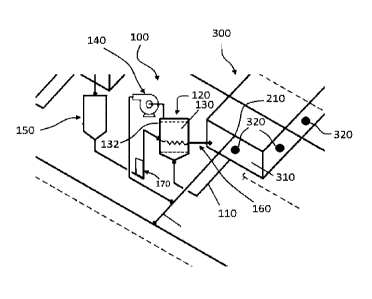
CA3082087 — DEVICE FOR COLLECTING AND REMOVING GASES IN AN ALUMINUM REDUCTION CELL — Obshchestvo s Ogranichennoy Otvetstvennosty yu “Obedinennaya Kompaniya Rusal Inzhenerno-Tekhnologicheskiy Tsentr” (Russia) — The invention relates to the production of aluminum in electrolysis cells with baked anodes. A device comprises a system of gas ducts and gas-collecting caps. Each of the caps is connected by a first channel to a horizontal gas duct to form a main gas removal circuit and is connected by a second channel to an additional gas duct to form an additional gas removal circuit. The height of each subsequent channel of the main loop is increased by 16-24% of the height of the preceding channel, and the height of each subsequent channel of the additional loop is increased by 24-26% of the height of the preceding channel. Separator plates are mounted inside the caps. The length of each subsequent plate is reduced with respect to the preceding by 25-35%. The technical effect is to reduce the volumes of gases removed from the electrolysis cell, and to maintain gas removal efficiency both between routine operations and during routine operations when reduction cells are unsealed, i.e. hooding covers are open.
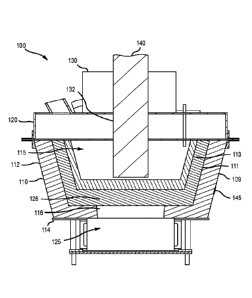
CA3075758 — METALLURGICAL SYSTEM INCLUDING A REFRACTORY VESSEL — Boston Electrometallurgical Corporation (USA) — The present technology relates to processing systems for performing molten oxide electrolysis or other metallurgical operations. Metallurgical assemblies and systems according to the present technology may include a refractory vessel including sides and a base. The base may define a plurality of apertures centrally located within the base. The sides and the base may at least partially define an interior volume of the refractory vessel. The assemblies may include a lid removably coupled with the refractory vessel and configured to form a seal with the refractory vessel. The lid may define a plurality of apertures through the lid. The assemblies may also include a current collector proximate the base of the refractory vessel. The current collector may include conductive extensions positioned within the plurality of apertures centrally located within the base.
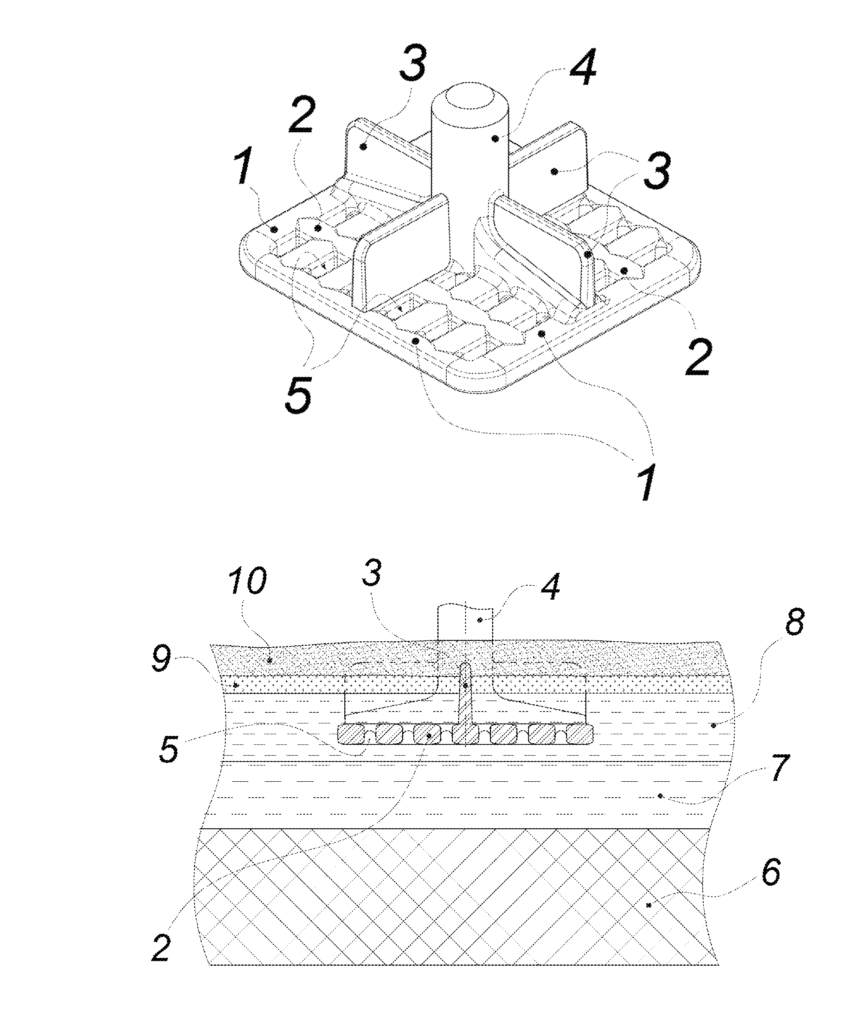
CA3054401 — METAL INERT ANODE FOR ALUMINUM PRODUCTION BY THE ELECTROLYSIS OF A MELT — Obshchestvo s Ogranichennoy Otvetstvennosty yu “Obedinennaya Kompaniya Rusal Inzhenerno-Tekhnologicheskiy Tsentr” (Russia) — One of the fundamental differences of inert anodes compared to carbon ones is that the diameter of oxygen bubbles released on inert anodes is significantly smaller than the diameter of bubbles of CO and CO2 released on carbon anodes. A design for a metallic inert anode is proposed which is configured in the form of a perforated structure having through holes formed by longitudinal and transverse anode elements that intersect with each other and are delimited by the lateral sides of the intersecting anode elements and contains vertical or slanted ribs that protrude from an electrolyte and are integrated with the anode elements or a current lead. As a result, the voltage drop in the anode and in the bubble layer under the anode is reduced, the anode voltage surge and anode consumption are reduced, and current efficiency and the reliability of a cryolite-alumina crust are increased, which leads to increased anode service life and helps form a strong and reliable cryolite-alumina crust on the surface of the melt, thus raising the efficiency of the process.
CA3043850 — METHOD OF ALUMINUM ALLOYS PRODUCTION — Obshchestvo s Ogranichennoy Otvetstvennosty yu “Obedinennaya Kompaniya Rusal Inzhenerno-Tekhnologicheskiy Tsentr” (Russia) — The invention relates to a method for producing aluminum-based alloys using electrolysis. The method comprises using a non-consumable anode of an aluminum electrolysis cell as a source of alloying elements, introducing alloying elements into a melted cathodic aluminum by dissolving the alloying elements from the non-consumable anode in an electrolyte melt of the aluminum electrolysis cell and/or by adding oxides and/or fluorides, and/or carbonites of alloying elements into the electrolyte melt of the aluminum electrolysis cell; reducing the alloying elements introduced into the electrolyte melt of the aluminum electrolysis cell on the melted cathodic aluminum, thus producing an aluminum alloy base; determining the element ratio in the aluminum alloy base; bringing the alloys to the specified formulation by adding alloying elements to the aluminum alloy base in the required quantities. The invention provides multicomponent aluminum alloys of a predetermined formulation by introducing alloying additives during electrolytic aluminum production followed by bringing the alloys to the specified formulation, consequently, the technology and control are simplified, the consumption of a master alloy is reduced, thus reducing the cost of the aluminum-based alloy production.
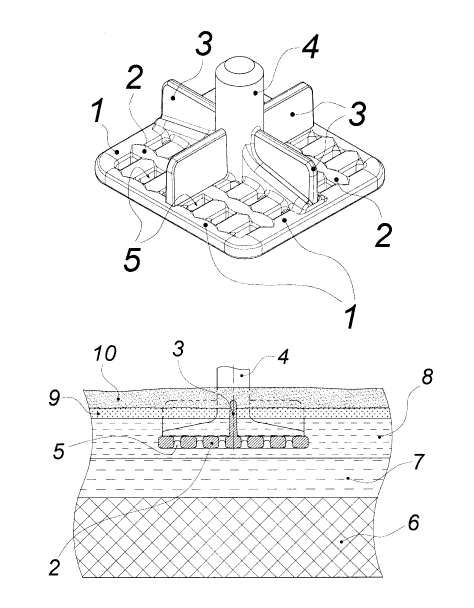
CA3031708 — CATHODE ASSEMBLY FOR THE PRODUCTION OF ALUMINUM — Tokai Cobex GMBH (Germany) — The present invention relates to a novel cathode assembly and its use for the production of aluminum in an electrolysis cell, with the cathode assembly for the production of aluminum comprising at least one cathode block on the basis of carbon and/or graphite, at least one current collector system of a highly electrically conductive material having an electrical conductivity greater than that of steel, wherein the terminal end parts of the at least one current collector system are extending outside of the at least one cathode block and/or are within the at least one cathode block characterized in that at least one part of the at least one current collector system is sloping upwards when viewed over the length of the cathode block, and wherein the highly electrically conductive material is copper or a copper alloy.
CA3030330 — ADVANCED ALUMINUM ELECTROLYSIS CELL — Elysis Limited Partnership (Canada) — It is disclosed an electrolytic cell including one anode module having a plurality of anodes (preferably oxygen-evolving anodes); one cathode module, opposing the anode module, having a plurality of vertical cathodes, wherein each of the anodes and cathodes being vertically oriented and spaced one from another; a cell reservoir; and a cell bottom supporting the cathode module. The cell bottom comprises upper surfaces and a channel, with the cathodes extending upward from the upper surfaces. The upper surfaces are configured to direct via gravity substantially all of the liquid aluminum produced in the electrolytic cell to the channel, and the channel is configured to receive liquid aluminum from the upper surfaces. The liquid aluminum produced at the outer surfaces of the cathodes flows via gravity therefrom across the upper surfaces and into the channel, thereby creating a flowing layer of liquid aluminum over the upper surfaces.
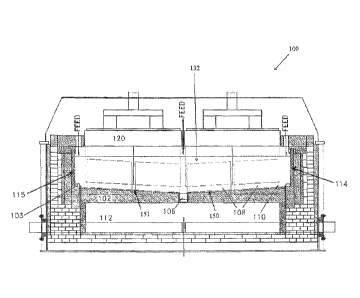
CA3030237 — ELECTROLYSIS CELL AND A METHOD FOR REPAIRING SAME — Norsk Hydro ASA (Norway) — An electrolysis cell of Hall-Héroult type for producing aluminum having prebaked anodes of a carbonaceous material, and a cathode structure comprising a pot shell made out of a steel material and further having a bottom and sides supported by structural elements such as cradles and beams, wherein the cathode structure further comprises a protective and insulating lining inside the shell and a carbon based cathodic bottom with collector bars embedded therein, and where produced aluminum metal forms a layer onto said cathodic bottom, an electrolytic bath positioned above said aluminum metal where the anodes are in electrical contact with said electrolyte, wherein at least part of the steel pot shell and/or deck plates are made of a ferritic stainless steel (FSS) material. The method relates to a repair procedure for replacing parts of a commonly used carbon steel material with ferritic stainless steel material at least in parts of the steel pot shell and/or deck plates heavily exposed to corrosion and detonation. The replacement procedure can either result in a homogenous or composite material structure in affected areas.
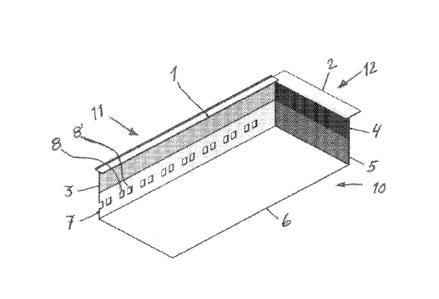
CA3012166 — METHOD FOR ESTIMATING DYNAMIC STATE VARIABLES IN AN ELECTROLYTIC CELL SUITABLE FOR THE HALL-HEROULT ELECTROLYSIS PROCESS — Dubai Aluminium PJSC (United Arab Emirates) Newsouth Innovations PTY Limited (Australia) — Method of producing aluminum in an electrolytic cell using the Hall-Héroult electrolysis process, said method comprising the following steps: -dividing said cell into at least n subsystems, -for each subsystem, estimating the local value of at least one target or output parameter on the basis of the value of the measurement of at least one input parameter, -modifying at least one of said input parameters in the cell by exerting a physical action upon the cell, if at least one estimated value of said output parameter, for at least one subsystem, is substantially different from another estimated value of said output parameter for another subsystem. This method is based on a particular approach of anode current measurement using digital communication for easy wiring, installation, and maintenance, and applies a Kalman filter type state observer to a dynamic model of the process for the estimation of unmeasured process variables.
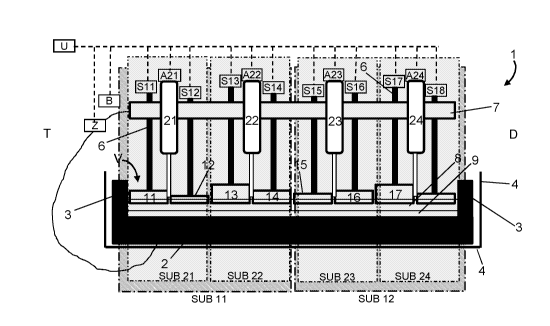
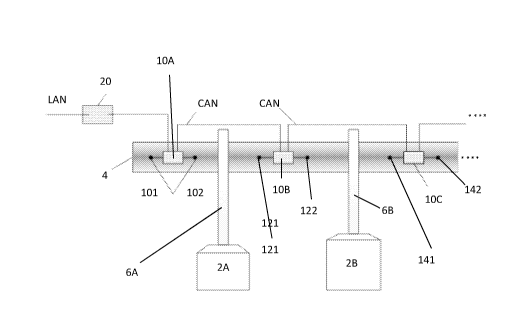
CA3012163 — METHOD OF MONITORING INDIVIDUAL ANODE CURRENTS IN AN ELECTROLYTIC CELL SUITABLE FOR THE HALL-HEROULT ELECTROLYSIS PROCESS — Dubai Aluminium PJSC (United Arab Emirates) Newsouth Innovations PTY Limited (Australia) — A method of anode current monitoring in an electrolytic cell suitable for the Hall-Héroult electrolysis process, said method comprising : – providing a plurality of sensing assemblies (10A, 10B, 10C) at a plurality of locations along the anode busbar, each sensing assembly comprising at least one sensing element (101, 102, 121, 122, 141, 142) and converting means, for converting a measured analog signal into a digital output, – measuring with at least one of said sensing element(s) at least one set of values of a representative parameter of current at at least one sensing time, – digitalizing said analog signals of values into digital outputs, using said converting means, said digital outputs representing the current flow in the anode beam in the vicinity of the sensing assembly having generated said digital output. This method allows to detect and monitor abnormal conditions of cell operation such as anode effects.
CA3007008 — IMPROVED METHOD FOR FABRICATING A DENSE, DIMENSIONALLY STABLE, WETTABLE CATHODE SUBSTRATE IN SITU — KCL Enterprises, LLC (USA) — Compositions suitable for use in an electrolytic cell for producing aluminum are provided. The compositions can contain a powder blend of boron oxide, titanium dioxide, aluminum, and titanium diboride. The powder blend can be compacted into tiles and arranged as a cathode surface. The boron oxide and the titanium dioxide in the tiles can be made to react under low temperature molten aluminum to produce titanium diboride in situ. The reaction yields a dense dimensionally stable wettable cathode substrate that can reduce the power consumption in the aluminum electrowinning process. For example, the composition may contain 3 molar equivalents of boron oxide, 3 molar equivalents of titanium dioxide, 7 to 21 molar equivalents of titanium diboride, and 20-40 molar equivalents of aluminum. Sintering of the compositions can be initiated using molten aluminum at a low temperature (for example about 700oC) after pressing into a tile at about 10 kpsi to 60 kpsi to form a cathode substrate in situ. The reaction of boron oxide, titanium dioxide, and aluminum is exothermic and can be detected by a spike in the aluminum bath temperature.
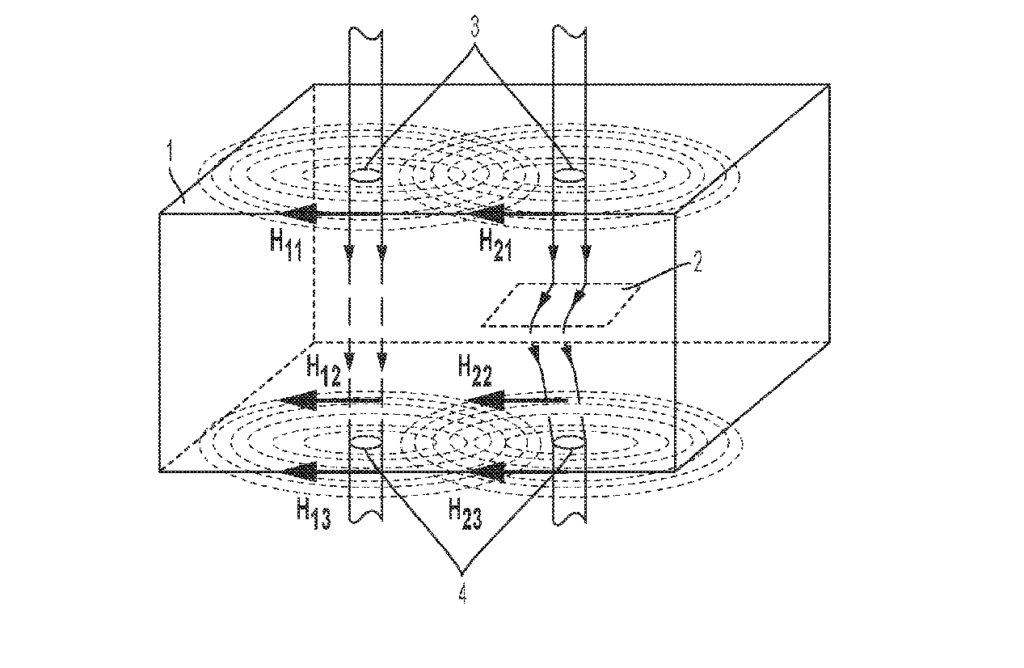
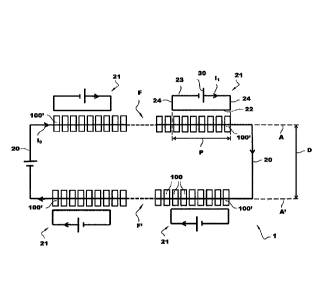
CA3000482 — SERIES OF ELECTROLYSIS CELLS FOR THE PRODUCTION OF ALUMINIUM COMPRISING MEANS FOR BALANCING THE MAGNETIC FIELDS AT THE END OF THE LINE — Rio Tinto Alcan International Limited (Canada) — The invention relates to a series (1) of electrolysis cells (100) intended for the production of aluminum, comprising: two rectilinear and parallel lines (F, F’) of electrolysis cells electrically connected in series, a connecting conductor (20) between a first end cell (100′) of one line and the corresponding first end cell (100′) of the other line, and at least one magnetic balancing circuit (21) for balancing the end of line cells, comprising a first electrical conductor (22) for magnetic balancing of the end cells extending along one of the lines of cells, only opposite an end portion (P) of the first line of cells.
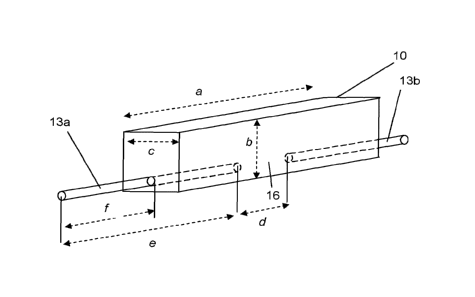
CA2980832 — CATHODE BLOCK FOR ELECTROLYTIC CELL SUITABLE FOR THE HALL-HEROULT PROCESS — Dubai Aluminium PJSC (United Arab Emirates) — Cathode element suitable for use in a Hall-Heroult electrolysis cell, comprising a cathode block (10) comprising a carbonaceous material, at least one metallic connection bar (13) made in copper or copper alloys, wherein said metallic connection bar (13) is fitted into a groove or bore in direct contact with a carbonaceous material, and wherein said carbonaceous material can be the carbonaceous material of said cathode block (10), or an intermediate carbonaceous material (17) that is in direct contact with the carbonaceous material of said cathode block (10).
CA2980248 — CERMET ELECTRODE MATERIAL — Elysis Limited Partnership (Canada) and Rio Tinto Alcan International Limited (Canada) — The invention relates to a cermet material comprising as mass percentages, at least: – 50% to 90% of a metallic phase (1 ) containing an alloy of copper (Cu) and nickel (Ni), – 10% to 50% of an oxide phase (2) containing at least iron, nickel and oxygen with the following proportion by mass of Ni: 0.2% < Ni= 17%, The invention also relates to an electrode, preferably an anode comprising said cermet material.

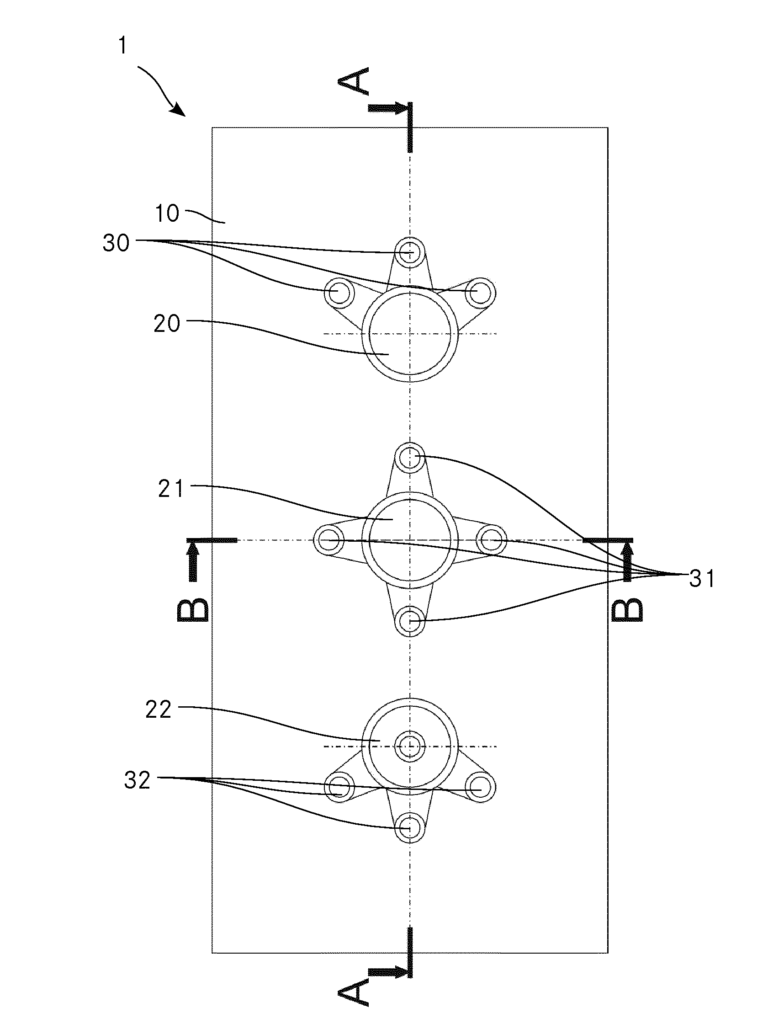
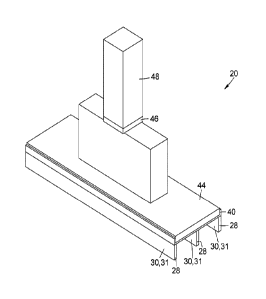
CA2976804 — ANODE ASSEMBLY FOR ALUMINUM ELECTROLYSIS CELLS AND METHOD FOR MANUFACTURING ANODE ASSEMBLIES — Universite du Quebec a Chicoutimi (Canada) — An anode assembly for an aluminum electrolysis cell is provided. The anode assembly includes a baked anode block, a plurality of elongated connection elements each having an anode block contact surface and an electrical connection surface, at least one electromechanical crossbar connector covering the electrical connection surfaces of the elongated connection elements, and a crossbar electrically connected to the elongated connection elements. A method for manufacturing an anode assembly for an aluminum electrolysis cell is also provided. The method includes the steps of forming a block of green anode paste, inserting a plurality of elongated connection elements in the green anode paste, baking the green anode, positioning a crossbar above the electrical connection surfaces of the plurality of elongated connection elements, and covering the electrical connection surfaces and at least partially the crossbar with a surface-conforming electrically conductive material.
CA2970605 — A MODIFIED ELECTROLYSIS CELL AND A METHOD FOR MODIFYING SAME — Norsk Hydro ASA (Norway) — The present invention relates to a method for reducing the metal pad unevenness and optimizing the MHD (magnet hydrodynamic) stability in an electrolysis cell for aluminum production, and a correspondingly modified cell. A method for optimizing stability in an electrolysis cell of the Hall-Héroult type where the cell has suspended prebaked anodes and a cathode panel. The panel comprises several cathode blocks or cathode block sections. A metal pad and an electrolytic bath are located between said anodes and the cathode panel. The force field acting on the metal pad is calculated and monitored in a computer-based model of the cell, whereby the local current paths and correspondingly the local forces in the metal above the cathode panel are modified by influencing selectively the current distribution in individual cathode blocks or block sections in the computer-based model. At least one modification is implemented in the cell.
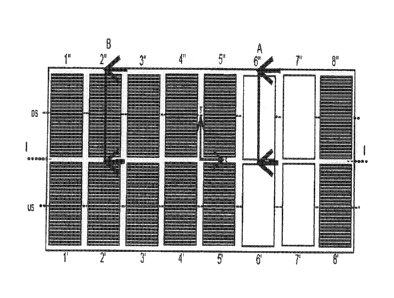
CA2975962 — ALUMINUM SMELTER AND METHOD TO COMPENSATE FOR A MAGNETIC FIELD CREATED BY THE CIRCULATION OF THE ELECTROLYSIS CURRENT OF SAID ALUMINIUM SMELTER — Rio Tinto Alcan International Limited (Canada) — The invention relates to an aluminum smelter (1) which comprises a row (2) of electrolysis cells arranged transversely relative to the row (2), one of the cells comprising anode assemblies and electrical conductors for raising and connecting to the anode assemblies. The raising and connection conductors extend upwards along two opposing longitudinal edges of the cell. In addition, the aluminum smelter (1) comprises a first electrical compensation circuit (4) extending under the cells and capable of carrying a first compensation current (IC1) in the opposite direction to the electrolysis current (IE), and a second electrical compensation circuit (6) extending on one side of the row (2) and being capable of carrying a second compensation current (IC2) in the same direction as the electrolysis current (IE).
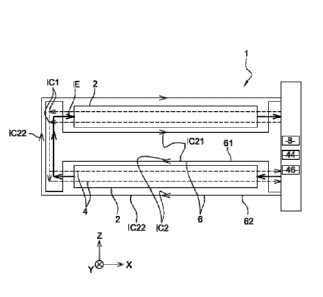
CA2975081 — AN ANODE FOR USE IN AN ELECTROLYSIS PROCESS FOR PRODUCTION OF ALUMINIUM IN CELLS OF HALL-HEROULT TYPE, AND A METHOD FOR MAKING SAME — Norsk Hydro ASA (Norway) — An anode for use in an electrolysis process for production of aluminum in cells of Hall- Héroult type, the anode comprises a body or block (120; 20) of calcinated carbonaceous material connected with an electrical current lead, where said current lead being connected with an anode rod (103; 3) and further being part of an anode hanger (101; 1). The current lead comprises at least one metallic suspension plate(s) (104; 4. 4′) with vertically oriented rodding plates (105 105”’,5, 5′) at least partly embedded by their lower partly in corresponding recesses (113, 113′”‘, 13. 13′; 100, 100’) in the top of the carbonaceous block (120; 20) and further connected by mechanical fixation means (108; 8; 14, 16). Said recesses are wider than the rodding plates and being filled with an electric conductive particulate material only. It Is also described a method for processing an undercut recess (10) in the anode top for mechanically fixing the anode block (20) to a protrusion (8) on the current lead.
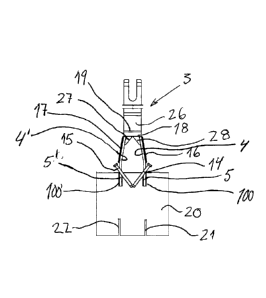
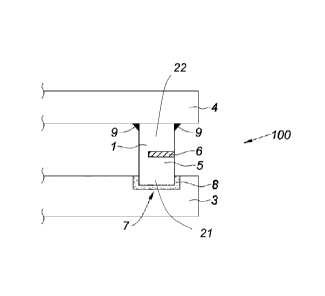
CA2952166 — ANODE ASSEMBLY — Rio Tinto Alcan International Limited (Canada) — The invention relates to an anode assembly (100) including an anode (3) and an anode holder (4) for producing aluminum, characterized in that the anode assembly (100) comprises an electrical connection element (1) for electrically connecting the anode holder (4) to the anode (3), and at least one thermally insulating element (6) arranged such as to reduce the heat transfer between the anode (3) and the anode holder (4) during the production of aluminum. The invention relates to an anode assembly (100) including an anode (3) and an anode holder (4) for producing aluminum, characterized in that the anode assembly (100) comprises an electrical connection element (1) for electrically connecting the anode holder (4) to the anode (3), and at least one thermally insulating element (6) arranged such as to reduce the heat transfer between the anode (3) and the anode holder (4) during the production of aluminum.