“The future cannot be predicted, but futures can be invented.”
— Denis Gabor, Nobelist and author of Inventing the Future (1963)
Editor’s Note: In the first of some 22 US patents granted to Charles Martin Hall for his invention of the aluminum electrolysis process, he basically claimed an inert anode “formed of copper, platinum, or other suitable non-carbonaceous material” (US 400,664 –granted on April 2, 1889). Only in a later patent (US 400,766 also granted on April 2, 1889) did he claim the use of a “carbonaceous anode”, which is universally used today worldwide. However, the search for an inert anode material that can replace prebaked or Søderberg carbon anodes, the consumption of which is the principal source of process related CO2 emissions from primary aluminum production, continues today. In fact, the International Patent Search published in our February 2020 issue focused on inert anode technology for aluminum electrolysis; it is worth a second look.
While the primary aluminum industry is still searching for an inert anode solution and must depend wholly on the use of “carbonaceous” anodes in their operations, steady progress is being made in optimizing aluminum electrolysis operation using carbon anodes. Several recent patents selected hereafter focus on carbon anodes in aluminum electrolysis, their composition, positioning within the reduction cell, cathode design, cryolite chemistry, and overall control of operation through innovative software and electronics. Taken together, these inventions minimize CO2 emissions and reduce the environmental consequences inherent in the Hall-Héroult process. Some of the ideas and solutions proposed here and in recent years for carbon anodes may apply to inert anodes as well and will provide the industry with even more environmentally-friendly aluminum.
— Joseph C. Benedyk, Editor
US11492716 — MATERIAL COMPONENTS PROTECTION AGAINST THE CORROSIVE ACTION OF CRYOLITE MELTS IN ALUMINUM REDUCTION CELLS — Laboratoire Cir Inc. (Canada) — Some elements contained in the molten electrolyte used in such cells can diffuse in the refractory materials and react therewith to reduce their effectiveness. This can shorten the useful lifetime of the cell, in addition to forming toxic compounds. The refractory materials thus require decontamination before disposal, at the end of the cell life. The present document describes an electrolytic cell comprising a protective layer comprising elemental copper covering at least in part or all of a refractory material assembly covering an interior surface thereof. Also described is a copper oxide containing composition comprising copper oxide and any one of a reducing agent and a binder. Also described is a method of protecting a refractory material assembly covering an interior surface of an electrolytic cell, comprising covering at least in part, or all of the refractory material assembly with a copper sheet, a structure comprising elemental copper, a copper oxide, an elemental copper comprising composite material, a copper oxide containing composition and combinations thereof, to provide a protective layer comprising elemental copper.
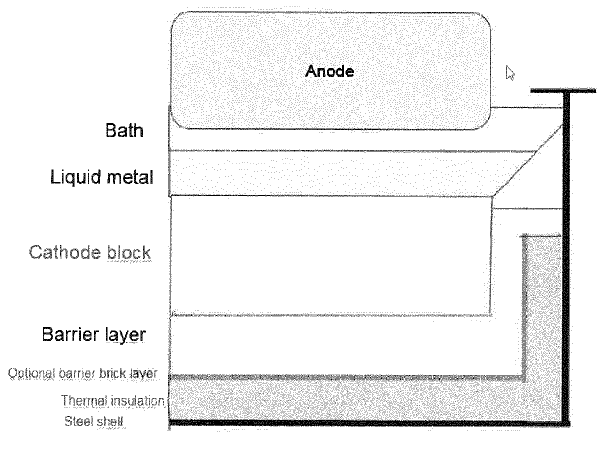
US11466377 — METHOD FOR PROVIDING A CATHODE LINING BARRIER LAYER IN AN ELECTROLYSIS CELL AND A MATERIAL FOR SAME — Norsk Hydro ASA (Norway) — The present invention relates to a method and a material for establishing a cathode barrier layer in electrolysis cells for production of aluminum of Hall-Heroult type, the barrier layer can comprise minerals combined with a compound that lowers the melting temperature of the minerals, such as fluorides. The benefits of the presented invention are linked to protection of the cell insulation. The new barrier is based on Na—Al—Si—O minerals such as high alkali feldspars or nepheline syenites (hereafter collectively denoted “minerals”) in combination with fluorides and SiO2. The minerals can be installed with the addition of 0.1-30 wt. % fluorides in the form of Na3AlF6 or its equivalent in electrolysis bath/NaF/AlF3 or spent pot lining. SiO2 can be used to adjust the viscosity of the mixture to the desired point. Importantly the barrier will form in the mineral layer below the cathode blocks and bath components will not be able to attack conventional barrier bricks or insulation bricks that is placed below the mineral layer.
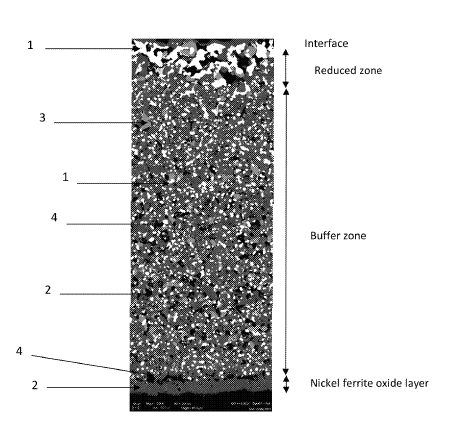
US11332837 — ELECTRODE MATERIAL AND USE THEREOF FOR THE MANUFACTURE OF AN INERT ANODE — Elysis Limited Partnership (Canada) — The invention relates to an electrode material, preferably an inert anode material comprising at least a metal core and a cermet material, characterized in that: said metal core contains at least one nickel (Ni) and iron (Fe) alloy, said cermet material comprises at least as percentages by weight: 45 to 80% of a nickel ferrite oxide phase (2) of composition NixFeyMzO4 with 0.60 ≤x≤0.90; 1.90≤y≤2.40; 0.00≤z≤0.20 and M being a metal selected from aluminum (Al), cobalt (Co), chromium (Cr), manganese (Mn), titanium (Ti), zirconium (Zr), tin (Sn), vanadium (V), niobium (Nb), tantalum (Ta) and hafnium (Hf) or being a combination of these metals, 15 to 45% of a metallic phase (1) comprising at least one alloy of nickel and copper. The new inert anode material used in the production of aluminum has satisfactory conductivity in the usual temperature conditions of igneous electrolysis processes, in order not to increase the electrical power consumption related to this technology; corrosion resistance in the cryolite baths usually used for this electrolysis, and which are aggressive; suitable mechanical properties of the inert anode with a view to industrial handling; a satisfactory life time of the inert anode from an industrial point of view (therefore essentially economic) and improved with respect to inert anodes known from prior art; reduction in contamination of the aluminum produced by electrolysis, and the electrolysis bath based on molten cryolite.
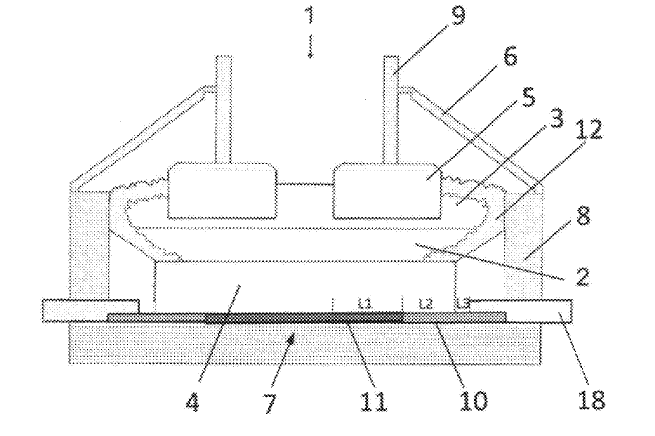
US11136682 — CATHODE CURRENT COLLECTOR FOR A HALL-HEROULT CELL — Novalum SA (Switzerland) — The invention is useful in the production of aluminum using the Hall-Héroult process for the optimization of the collector bars to decrease energy consumption, maximization of the current efficiency, and increase cell productivity. The invention relates to an electrolytic cell (1) for production of aluminum (2) including collector bars structure modifications (13,14,15,16) under the cathode (4), namely a copper collector bar held in a U-shaped profile or directly embedded into the cathode. This leads to an optimized current distribution in the liquid aluminum metal (2) and/or inside the carbon cathode allowing for operating the cell at lower voltage. The lower voltage results from either a lower anode to cathode distance (ACD), and/or to lower voltage drop inside the carbon cathode from liquid metal to the end of the collector bar.
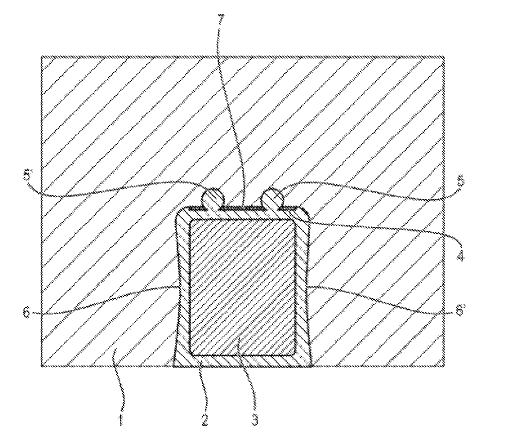
US11339490 — ALUMINUM ELECTROLYZER ELECTRODE (VARIANTS) — United Company RUSAL Engineering and Technology Centre LLC (Russia) — The invention relates to vertical or inclined electrodes of an electrolyzer for electrolytically producing aluminum from aluminum oxide. An electrode contains an electrode base and a surface coating based on refractory ceramics. According to a first variant of the invention, the electrode base is made of a composite material containing between 5% and 90% by mass of refractory ceramics, and of at least one metal having a melting temperature exceeding 1000°C., which forms refractory intermetallic compounds upon interaction with aluminum, and/or containing at least one alloy of such a metal. According to a second variant of the invention, the electrode base is made of a metal alloy, for example structural steel or another alloy, and the surface of the electrode base has applied thereto an intermediary layer consisting of a composite material having the composition described above. The use of vertical or inclined wetted cathodes, as well as of bipolar electrodes in new aluminum electrolyzers reduces specific rated power consumption for aluminum production by means of reduction of an anode-to-cathode distance (ACD) between electrodes and improves electrolyzer performance on a per occupied area basis.
US11339489 — CATHODE BLOCK HAVING A SLOT GEOMETRY — Tokai COBEX GmbH (Germany) — Present-day cathode bottoms are subject to wear. As a result of the aluminum layer moving over the cathode bottom, mechanical wear of the cathode surface takes place. Additionally, through aluminum carbide formation and sodium incorporation, (electro)chemical corrosion of the cathode bottom takes place. The failure and replacement of a cathode block in an electrolysis cell in such a plant can be expensive and require complex repairs which severely reduce the profitability of the plant. Disclosed is a cathode block having a slot geometry, said carbon-based cathode block for an electrolysis cell for the production of aluminum being provided with at least one slot for accommodating at least one cathode bar, said at least one slot having at least one cavity, at least some sections of which extend in the slot direction and which includes at least one undercut. The intermediate spaces between the individual walls of the cathode blocks delimiting defining the slots and the cathode bar are normally cast from cast iron, by means of which a cast iron-cathode bar combination is formed. Through this filling of the slot, it is ensured that during operation, the cathode bar is connected electrically and mechanically to the cathode block. Following this process step, the assembly consisting of cathode bar, cast iron and cathode block is rotated by 180° into what is known as the operating position where the slot opening for the cathode bar points downward. This is then brought to an operating temperature of approximately 960°C. and then continuously operated for several years.
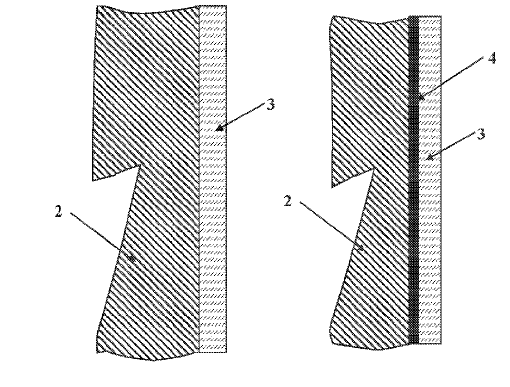
US11286574 — CATHODE CURRENT COLLECTOR/CONNECTOR FOR A HALL-HEROULT CELL — Tokai COBEX GmbH (Germany) — An electrolytic cell for aluminum production including collector bars under the cathode, namely a copper collector bar whose external terminal end is connected by a conductor element providing electrical connection of the collector bar to an external bus. This conductor element comprises a flexible connector strip of the same or a different highly conductive metal as the conductor bar, such as copper. The main and first object of the invention is to simplify the collector bar system by using the copper bar (or copper bars) as one piece going from inside the carbon cathode directly outside the cell where it is connected at the position where a steel bar was formerly connected. The invention enables to dispense advantageously with the former steel connector bars, at lower cost, by providing connections that are reliable over the long term, reducing the heat flux and with a lower overvoltage penalty.
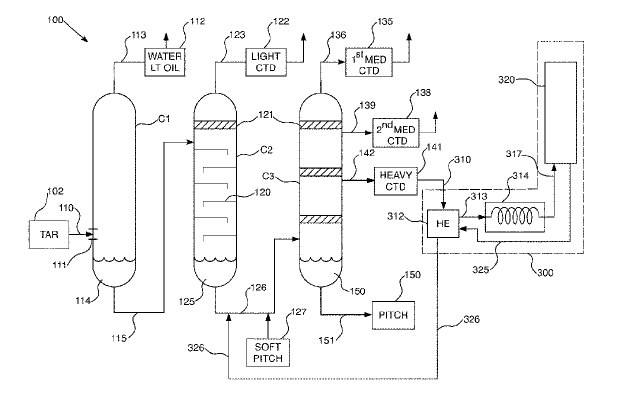
US11248172 — HEAT TREATMENT PROCESS AND SYSTEM FOR INCREASED PITCH YIELDS — Koppers Delaware, Inc. (USA) — Pitch production systems utilizing coal tar or decant oil for coal or petroleum based pitch are disclosed. Total pitch production yields are increased by heat treating distillate fractions from the pitch production process. A heat treatment system and process are disclosed in embodiments. The heaviest distillates having the highest molecular weights are subjected to heat treatment, though other embodiments contemplate heat treating a variety of combined distillate fractions. The heat treatment systems require heat soaking the distillate(s) at elevated temperatures of 459-535°C at a near-constant temperature with near-uniform flow. A fraction of the heat-treated distillate may be reintroduced to the pitch production system as part of a continuous process. A significant fraction of the distilled coal tar material is coal tar pitch residue. This material is utilized in the production of anodes for aluminum smelting, as well as electrodes for electric arc furnaces used in the steel industry.
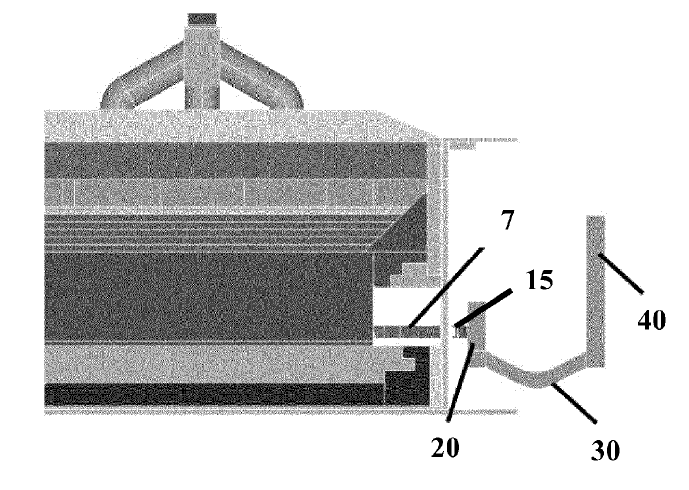
US11242604 — CATHODE ASSEMBLY FOR THE PRODUCTION OF ALUMINUM — COBEX GmbH (Germany) — A novel cathode assembly and its use aluminum production in an electrolysis cell. It is the object of the present invention to provide a cathode assembly which dispenses with cast iron or carbonaceous ramming paste and which can be directly connected to the external bus bar system, i.e., which can be directly installed in the electrolysis cell upon delivery. Furthermore, this cathode assembly should provide a more homogeneous current distribution within the cathode blocks and a reduced voltage drop. According to the present invention, this object is solved by a cathode assembly for the production of aluminum comprising at least one cathode block on the basis of carbon and/or graphite, at least one current collector system of a highly electrically conductive material having an electrical conductivity greater than that of steel, wherein the terminal end parts of the at least one current collector system are extending outside of the at least one cathode block and/or, preferably or, are within the at least one cathode block characterized in that at least one part, preferably all parts, of the at least one current collector system is/are sloping upwards when viewed over the length of the cathode block.
US11225725 — METHOD FOR PRODUCING ALUMINUM — UACJ Corporation (Japan) — The present disclosure relates to an inexpensive and environmentally friendly method for producing aluminum. A method for producing aluminum includes: a dissolution step of dissolving a hydrate containing Al in water to prepare an aqueous solution that contains Al ions; an extraction step of bringing an organic phase that is composed of an extractant into contact with an aqueous phase that is composed of the aqueous solution to extract the Al ions in the aqueous phase into the organic phase; and an electrodeposition step of electrolyzing the organic phase as an electrolytic solution to electrodeposit metallic Al onto a surface of a cathode from the Al ions in the electrolytic solution. The cathode may include metallic materials such as platinum, iron, copper, titanium, nickel and carbon, and plastic materials to which conductivity is imparted. In addition, as for the anode, aluminum can be used if the anode is a soluble anode, and carbon or the like can be used it the anode is an insoluble anode.
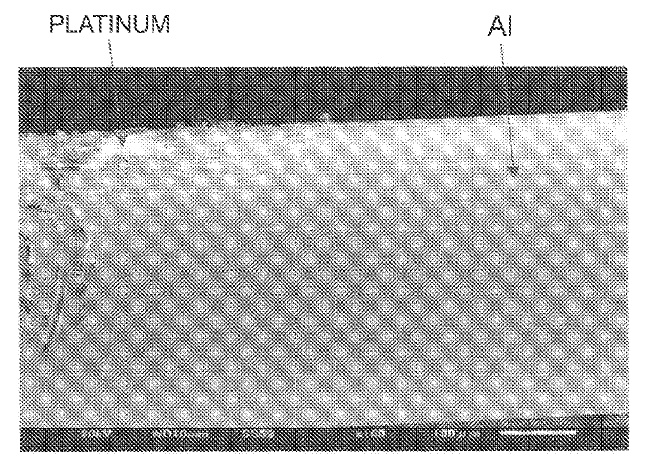
US11203814 — APPARATUSES AND SYSTEMS FOR VERTICAL ELECTROLYSIS CELLS — Alcoa USA Corp. (USA) — Broadly, the present disclosure relates to vertical cell electrode assemblies, in which both anodes and cathodes are configured in a vertical, alternating parallel configuration. More specifically, the present disclosure relates to vertical cell electrode assemblies, including the cathode support assembly/apparatus which is configured to retain the cathode(s) in the cell bottom in a substantially vertical configuration. Various ones of the inventive aspects noted hereinabove may be combined to yield electrolysis cells, cathode supports, and methods of making aluminum in an electrolysis cell having vertical cell configurations. In one embodiment, the disclosed subject matter relates to an electrolytic cell that has: a cell reservoir; a cathode support retained on a bottom of the cell reservoir, wherein the cathode support contacts at least one of: a metal pad and a molten electrolyte bath within the cell reservoir, wherein the cathode support includes: a body having a support bottom, which is configured to be in communication with the bottom of the electrolysis cell; and a support top, opposite the support bottom, having a cathode attachment area configured to retain a at least one cathode plate therein.
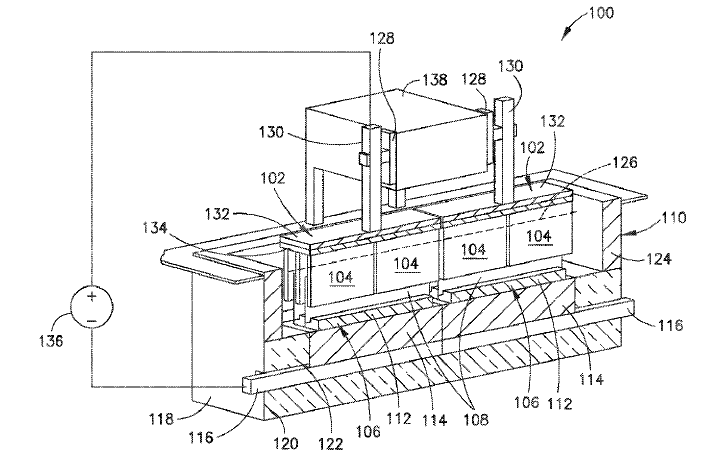
US11180862 — ADVANCED ALUMINUM ELECTROLYSIS CELL — Elysis Limited Partnership (Canada) — In some embodiments, an electrolytic cell includes: an one anode module having a plurality of anodes; a one cathode module, opposing the anode module, and comprising a plurality of vertical cathodes, wherein each of the plurality of anodes and each of the plurality of vertical cathodes are vertically oriented and spaced one from another; a cell reservoir; and a cell bottom supporting the cathode module, wherein the cell bottom comprise an first upper surface, a second upper surface, and a channel, wherein the plurality of vertical cathodes extends upward from the upper surfaces, wherein at least one cathode block is located below the plurality of vertical cathodes, wherein the first upper surface and the second upper surface are configured to direct substantially all of the liquid aluminum produced in the electrolytic cell to the channel, and wherein the channel is configured to receive liquid aluminum from the upper surfaces.
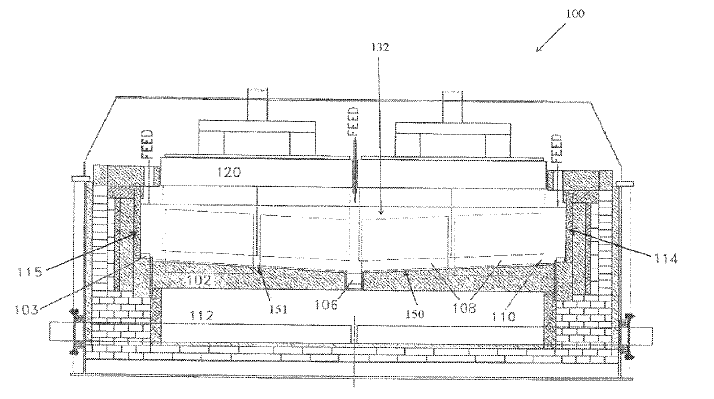
US11136682 — CATHODE CURRENT COLLECTOR FOR A HALL-HEROULT CELL — Novalum SA (Switzerland) — The invention relates to an electrolytic cell (1) for aluminum production (2) including collector bars structure modifications (13,14,15,16) under the cathode (4), namely a copper collector bar held in a U-shaped profile or directly embedded into the cathode. This leads to an optimized current distribution in the liquid aluminum metal (2) and/or inside the carbon cathode allowing for operating the cell at lower voltage. The lower voltage results from either a lower anode to cathode distance (ACD), and/or to lower voltage drop inside the carbon cathode from liquid metal to the end of the collector bar.

US11339490 — ALUMINUM ELECTROLYZER ELECTRODE (VARIANTS) — United Company RUSAL Engineering and Technology Centre LLC (Russia) — The invention relates to vertical or inclined electrodes of an electrolyzer for electrolytically producing aluminum from aluminum oxide. An electrode contains an electrode base and a surface coating based on refractory ceramics. According to a first variant of the invention, the electrode base is made of a composite material containing between 5% and 90% by mass of refractory ceramics, and of at least one metal having a melting temperature exceeding 1000°C, which forms refractory intermetallic compounds upon interaction with aluminum, and/or containing at least one alloy of such a metal. According to a second variant of the invention, the electrode base is made of a metal alloy, for example structural steel or another alloy, and the surface of the electrode base has applied thereto an intermediary layer consisting of a composite material having the composition described above. For this purpose, such electrodes must exhibit high electrical conductivity, mechanical strength, crack resistance and they must be resistant to aluminum and molten electrolyte impact at 1000°C. The use of vertical or inclined wetted cathodes, as well as of bipolar electrodes in new aluminum electrolyzers reduces specific rated power consumption by means of reduction of an anode-to-cathode distance (ACD) between electrodes and improves electrolyzer performance on a per occupied area basis.

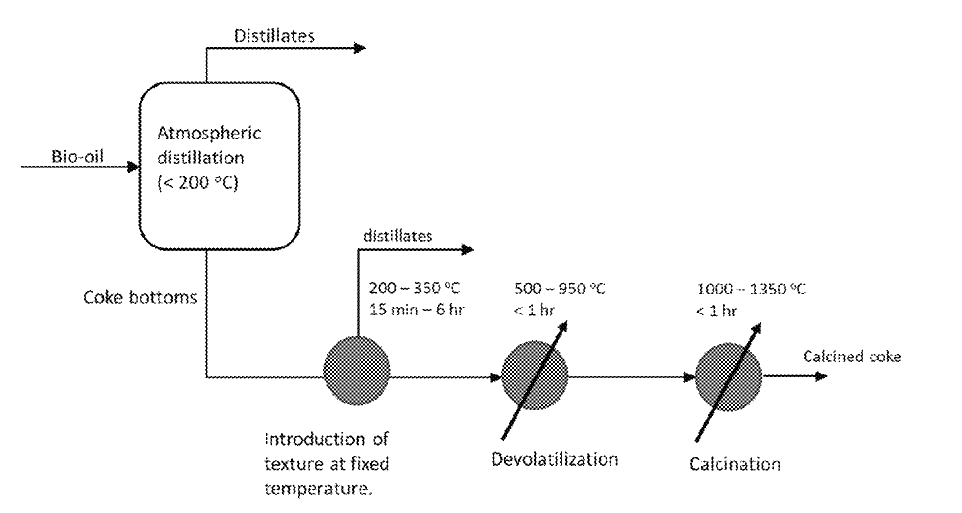
US11060033 — COMPOSITIONS AND METHODS FOR PRODUCING CALCINED COKE FROM BIORENEWABLE SOURCES — The United States of America, as represented by The Secretary of Agriculture (USA) and Rio Tinto Alcan Inc. (Canada) — Typically, residuals remaining after vacuum distillation of petroleum are sent to a delayed coker unit, which thermally cracks the residuals into ”petcoke” and lighter fuel components. Petcoke is sometimes used as a fuel without further refining or, if the metals and sulfur contents are low enough, calcined into coke suitable for use in other applications such as, for example, aluminum smelting anodes, steel carburization, and titanium dioxide production. Aluminum smelting alone may absorb more than 70% of the petcoke market. Carbon anodes for the industrial process of smelting aluminum, for example, are typically made of non-renewable materials, such as petcoke and coal tar pitch, which creates a substantial carbon footprint. One option to reduce this carbon footprint is to use renewable, biomass-derived anode raw materials. The disclosed invention relates generally to novel and improved methods of making coke products from biomass-derived oils. More specifically, the invention relates to methods of synthesizing biomass-derived calcined coke having desirable structures and characteristics from byproducts of bio-oil distillation. Disclosed are methods of producing calcined coke from bio-oil from a biomass feedstock. Also disclosed are calcined cokes produced by such methods having desirable structural characteristics.
US10975484 — ELECTROLYTE FOR OBTAINING MELTS USING AN ALUMINUM ELECTROLYZER — United Company RUSAL Engineering and Technology Centre LLC (Russia) — The invention concerns non-ferrous metallurgy, in particular the composition of an electrolyte for electrically obtaining aluminum by the electrolysis of fluoride melts. The electrolyte proposed contains, in % by weight: sodium fluoride 26-43, potassium fluoride up to 12, lithium fluoride up to 5, calcium fluoride 2-6, alumina 2-6, aluminum fluoride and admixtures—the remainder. The technical result is to increase the solubility of alumina in the electrolyte at a temperature of 830-930°C In the electrolyte being applied for, the carbon and inert electrode materials are not destroyed, and the use of special methods to purify the aluminum of melt components is not required. The task of the invention is the increase productivity and to reduce the cost price of obtaining aluminum at an electrolysis temperature of 830-930°C.
US10920329 — ANODE ASSEMBLY FOR ALUMINUM ELECTROLYSIS CELLS AND METHOD — Université Du Québec à Chicoutimi (Canada) — An anode assembly for an aluminum electrolysis cell is provided. The anode assembly includes a baked anode block, a plurality of elongated connection elements each having an anode block contact surface and an electrical connection surface, at least one electromechanical crossbar connector covering the electrical connection surfaces of the elongated connection elements, and a crossbar electrically connected to the elongated connection elements. A method for manufacturing an anode assembly for an aluminum electrolysis cell is also provided. The method includes the steps of forming a block of green anode paste, inserting a plurality of elongated connection elements in the green anode paste, baking the green anode, positioning a crossbar above the electrical connection surfaces of the plurality of elongated connection elements, and covering the electrical connection surfaces and at least partially the crossbar with a surface-conforming, electrically conductive material.
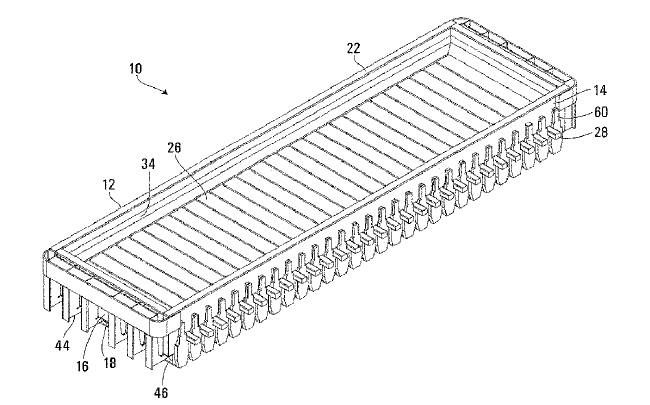
US10889906 — LOW-PROFILE ALUMINUM CELL POTSHELL AND METHOD FOR INCREASING THE PRODUCTION CAPACITY OF AN ALUMINUM CELL POTLINE — Hatch Ltd. (Canada) — The present invention relates to a method for increasing the reactive area within an existing potshell footprint to increase the productivity or lower the capital costs/tonne production capacity of an aluminum Hall-Heroult cell potline. An aluminum reduction cell having a shell structure with a pair of longitudinally extending sidewalls, a pair of transversely extending endwalls, a bottom wall, and an open top having an upper edge. The aluminum reduction cell also has a transverse support structure with transverse bottom beams located under the shell structure and extending transversely between the sidewalls, each of the transverse bottom beams having a pair of opposed ends. The aluminum reduction cell also has compliant binding elements fixed to the transverse support structure, each extending vertically along an outer surface of one of the sidewalls for applying an inwardly directed force said sidewall. The compliant binding elements are in the form of cantilever springs. Each spring has a metal member with a lower end which is secured to the transverse support structure, and a compliant, upper free end which is movable inwardly and outwardly in response to expansion and contraction of the shell structure.
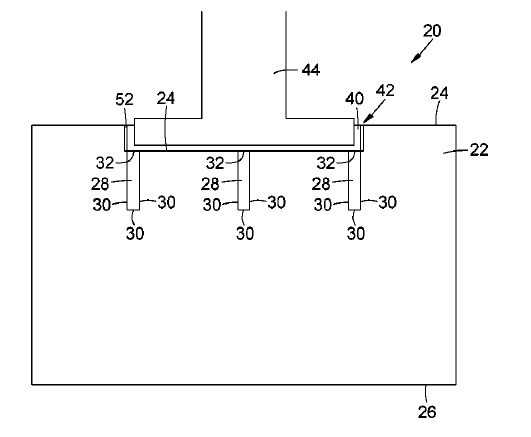
US10855040 — FLEXIBLE ELECTRICAL CONNECTORS FOR ELECTROLYTIC CELLS — Hatch Ltd. (Canada) — A flexible electrical connector assembly is adapted to connect a bus bar of an electrolytic cell to a collector bar of the electrolytic cell. The assembly includes an electrical connector including a plurality of conductive metal sheets, the electrical connector having a collector bar end and a bus bar end. The electrical connector may be adapted for being joined, at the collector bar end, to the collector bar and, at the bus bar end, to the bus bar. The electrical connector may be adapted to implement a change in direction, at a bend along a current-carrying path between the bus bar end and the collector bar end, the bend assisting to define the change in direction as greater than 90 degrees. Aspects of the present application relate to a flexible electrical connector that allows for an increase in the reaction area for a given electrolytic cell footprint. Conveniently, such an increase may be considered to increase a production capacity and/or lower capital costs per tonne of production capacity of an aluminum Hall-Héroult cell potline.
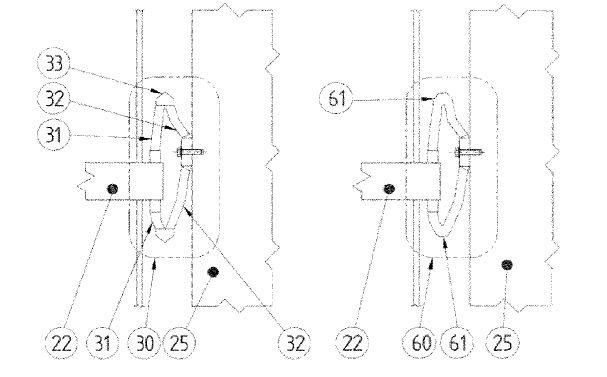
US10697074 — ELECTROLYTIC CELL INTENDED FOR THE PRODUCTION OF ALUMINIUM AND ELECTROLYTIC SMELTER COMPRISING THIS CELL — Rio Tinto Alcan International Limited (Canada) — This cell (1) comprises a pot shell (2) having two longitudinal sides (18) which are symmetrical in relation to a longitudinal median plane (P) of the electrolytic cell (1), an anode assembly which can only move in vertical translation with respect to the pot shell (2), the anode assembly comprising an anode block (100) and a transverse anode support (200) extending at right angles to the longitudinal sides (18) of the pot shell (2), from which support the anode block (100) is suspended. The anode support (200) comprises two connecting portions (202) from which electrolysis current is supplied to the anode support (200), and the cell (1) comprises electrical connection conductors (20) electrically connected to the two connecting portions (202) of the anode support (200), the two connecting portions (202) being located on either side of the plane (P).
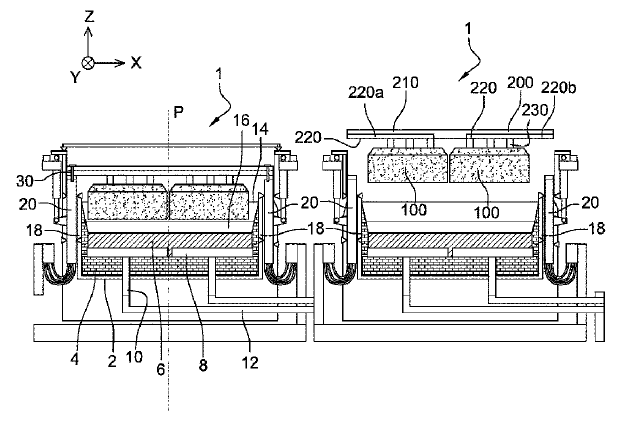
US10689770 — MODIFIED ELECTROLYSIS CELL AND A METHOD FOR MODIFYING SAME — Norsk Hydro ASA — The present invention relates to a method for reducing the metal pad unevenness and optimizing the MHD (magnet hydrodynamic) stability in an electrolysis cell of the Hall-Héroult type for aluminum production, and a correspondingly modified cell. A method for optimizing stability in an electrolysis cell of the Hall-Héroult type where the cell has suspended prebaked anodes and a cathode panel. The panel comprises several cathode blocks or cathode block sections. A metal pad and an electrolytic bath are located between said anodes and the cathode panel. The force field acting on the metal pad is calculated and monitored in a computer-based model of the cell, whereby the local current paths and correspondingly the local forces in the metal above the cathode panel are modified by influencing selectively the current distribution in individual cathode blocks or block sections in the computer-based model. At least one modification is implemented in the cell. The invention also relates to a correspondingly modified cell.
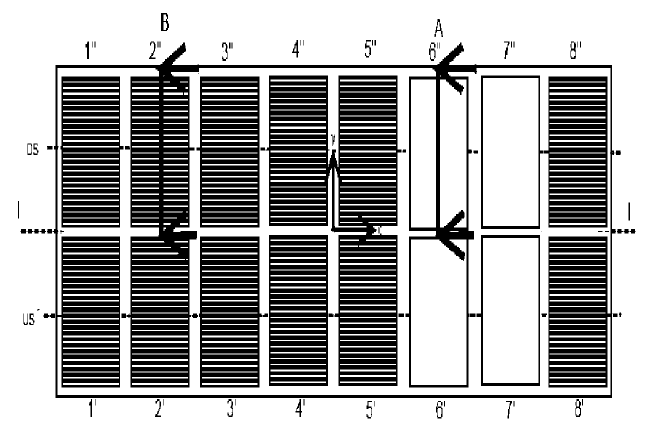
US10627787 — MANUFACTURING PROCESS CONTROL BASED ON MULTI-MODALITY AND MULTI-RESOLUTION TIME SERIES DATA — International Business Machines Corporation (USA) — The present invention relates generally to the field of optimizing manufacturing processes, and more particularly to optimizing aluminum smelting manufacturing based on multi-modality and multi-resolution time series data. Embodiments of the present invention disclose a method, a computer program product, and a system for optimizing aluminum smelting control. Calculating, by the one or more processors, an indicator function. Estimating, by the one or more processors, a coefficient vector based on the indicator function. Updating, by the one or more processors, the coefficient vector. Determining, by the one or more processors, if a change in the coefficient vector is less than a predetermined value, and responsive to determining the change in the coefficient vector is less than the predetermined value, outputting, a target time series for controlling aluminum smelting. Determining if a change in the coefficient vector is less than a predetermined value, and responsive to determining the change in the coefficient vector is less than the predetermined value, outputting a target time series for controlling aluminum smelting.
US10560987 — ELECTRODE COMPOSITION — Rheinfelden Carbon GmbH & Co. KG (Germany) — The present invention relates to Söderberg electrodes of an electric arc furnace, and to a baked electrode produced from the self-calcining electrode composition., containing one or more carbon components and a binder, wherein the binder is hard bitumen and having a needle penetration at 25°C. according to DIN EN 1426 of <50 [per 0.1 mm] and/or a softening point (ring and ball) according to DIN EN 1 427 of at least 65°C. and/or having a density at 25°C. according to DIN EN 52004 of 0.5 to 2 g/cm3, wherein the electrode material has a PAH content of <500 ppm. The hard bitumen is preferably derived by flash distillation from soft and medium-hard bitumen types and has a high sulfur content.
US10480089 — ANODE ASSEMBLY AND ASSOCIATED PRODUCTION METHOD — Rio Tinto Alcan International Limited (Canada) — The present invention relates to a manufacturing process for an anode assembly intended for cells for production of aluminum by electrolysis and particularly suited to electrolytic cells with pre-baked anodes. the anode assembly being of the type having an anode rod, a longitudinal member interdependent with one end of the anode rod and a carbon anode including a cavity in which is housed the longitudinal member, the method comprising a formation phase of at least one sealed area filled with sealing material and at least one unsealed area devoid of sealing material, said at least one unsealed area extending to one of the longitudinal ends of the longitudinal member.
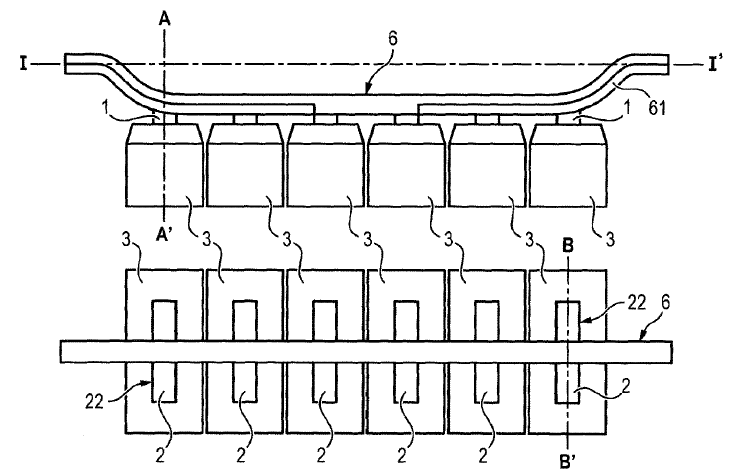
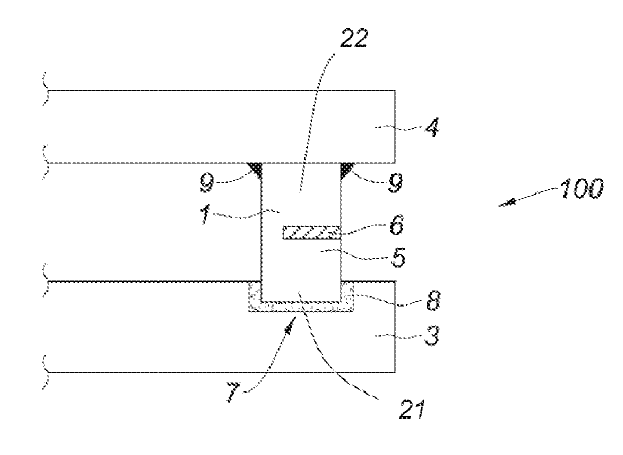
US10443140 — ANODE ASSEMBLY — Rio Tinto Alcan International Limited (Canada) — Anode assembly (100) comprising an anode (3) and an anode support (4) for the production of aluminum, characterized in that the anode assembly (100) comprises an electrical connecting element (1) to electrically connect the anode support (4) with the anode (3), and at least one thermally insulating element (6) arranged to reduce heat transfer between the anode (3) and the anode support (4) during the production of aluminum. The invention therefore aims to propose a device to limit heat losses without affecting its electrical conductance while minimizing costs. To do this, the invention provides an anode assembly for the production of aluminum comprising an anode, an anode support, and an electrical connecting element having a sealing portion and a non-sealing portion for electrically connecting the anode support to the anode, wherein the anode comprises a recess in which is housed the sealing portion of the electrical connecting element and wherein a seal formed of an electrically conductive material holds the electrical connecting element, the anode assembly comprising at least one thermally insulating element arranged between two walls facing each other belonging to the non-sealing portion of the electrical connection element and/or the anode support to reduce heat transfer between the anode and the anode support during the production of aluminum.
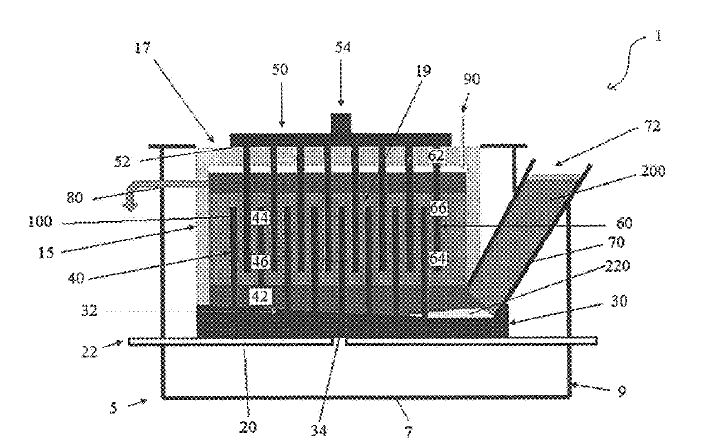
US10407786 — SYSTEMS AND METHODS FOR PURIFYING ALUMINUM — Alcoa USA Corp. — The application is directed towards methods for purifying an aluminum feedstock material. A method provides: (a) feeding an aluminum feedstock into a cell (b) directing an electric current into an anode through an electrolyte and into a cathode, wherein the anode comprises an elongate vertical anode, and wherein the cathode comprises an elongate vertical cathode, wherein the anode and cathode are configured to extend into the electrolyte zone, such that within the electrolyte zone the anode and cathode are configured with an anode-cathode overlap and an anode-cathode distance; and producing some purified aluminum product from the aluminum feedstock. In some embodiments, the method includes: the purified aluminum product comprises an aluminum purity to 99.999 wt. % Al.
US10358733 — ALUMINUM SMELTER AND METHOD TO COMPENSATE FOR A MAGNETIC FIELD CREATED BY THE CIRCULATION OF THE ELECTROLYSIS CURRENT OF SAID ALUMINUM SMELTER — Rio Tinto Alcan International Limited (Canada) — This aluminum smelter comprises a line of electrolytic cells arranged transversely to the line, one of the cells comprising anode assemblies and electrical conductors mounted and connecting the anode assemblies. Rising and connecting conductors extend upwardly along two opposite longitudinal edges of the cell. In addition, the aluminum smelter comprises a first electrical compensating circuit extending under the cell and which can be traversed by a first compensating current in the opposite direction to that of the electrolysis current, a second electrical compensating circuit extending on one side of the line that can be traversed by a second compensating current in the same direction as the electrolysis current.
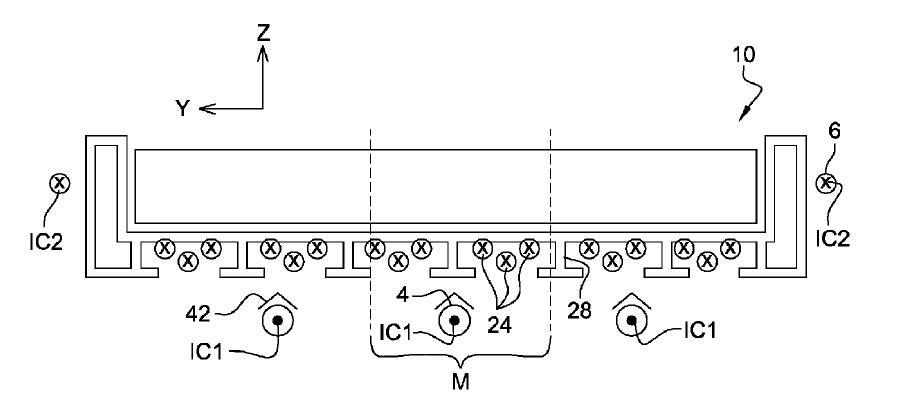
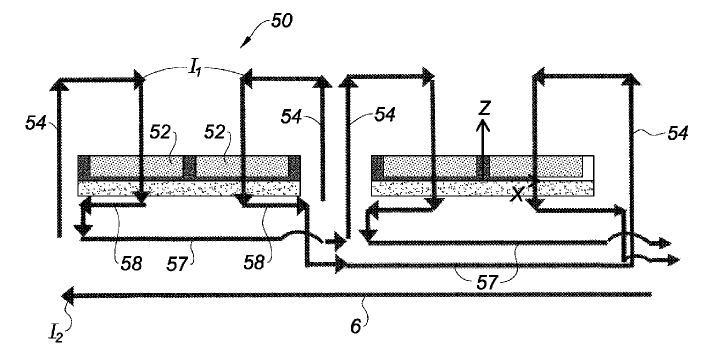
US10344390 — ALUMINUM SMELTER COMPRISING A COMPENSATING ELECTRIC CIRCUIT — Rio Tinto Alcan International Limited (Canada) — The aluminum smelter according to the invention uses up less space and offers the advantage that it can have cells which are magnetically very stable, to the extent that overall performance is improved. This aluminum smelter comprises a row of cells (50) arranged transversely in relation to the length of the row, the cells (50) individually comprising an anode (52), rising and connecting electrical conductors (54) running upwards along the two opposite longitudinal edges of the cell (50) to route the electrolysis current towards the anode (52), and a cathode (56) through which pass cathode conductors (55) connected to cathode outputs connected to linking conductors to route the electrolysis current to the rising and connecting electrical conductors of the next cell (50). Furthermore, the aluminum smelter comprises a compensating electrical circuit separate from the electrical circuit through which the electrolysis current flows, running beneath the cells (50), through which a compensating current may flow beneath the cells (50) in a direction opposite to the overall direction of flow of the electrolysis current.
US10309021 — METHOD FOR PREPARING AN ELECTROLYTE AND AN ELECTROLYTE REPLENISHMENT SYSTEM DURING ALUMINUM ELECTROLYSIS PROCESS — Shenzhen Sunxing Light Alloys Materials Co., Ltd. (China) — The disclosure provides a method for preparing an electrolyte and an electrolyte replenishment system during an electrolytic process. The method includes the following steps: Step A: placing aluminum in a reactor, vacuumizing the reactor and feeding an inert gas, heating the reactor to 700-850°C, and adding one or more of potassium fluozirconate, potassium fluoborate, sodium hexafluorozirconate and sodium fluoroborate; and Step B: stirring the reactants for 4-6 hours and extracting the upper molten liquid to obtain an electrolyte replenishment system during an aluminum electrolysis process. The disclosure has the following beneficial effects: when used in the aluminum electrolysis industry, the electrolyte system provided herein can be directly used as an aluminum electrolyte or a replenishment system in an electrolyte without changing existing electrolysis technology to significantly reduce an electrolysis temperature during an aluminum electrolysis process.
US10246790 — ALUMINUM ELECTROLYSIS CELL CATHODE SHUNT DESIGN — United Company Rusal Engineering and Technology Centre LLC (Russia) — The invention relates to electrowinning of aluminum from cryolite-alumina melts and can be used in the shunt design of a cathode assembly. In an aluminum electrolysis cell, cathode vertical metal shunts, are designed such that their top part is melted aluminum, and the bottom part is solid aluminum. Shunts are located in conduits made in a hearth slab lining which has a widening in the middle part which is wider than both parts of the shunts. The widening in the shunt conduit can be filled with a composite material, i.e., titanium diboride-carbon. The shunts can be designed as a tube, and the widening in the conduit and the space inside the tube can be filled with the composite material titanium diboride-carbon. The invention makes it possible to increase the electrical efficiency due to the absence of contact assemblies, reduced current loss, and achieving an effective current distribution and current shunting.
US10202701 — POTASSIUM CRYOLITE FOR ALUMINUM ELECTROLYSIS INDUSTRY AND PREPARATION METHOD THEREOF — Shenzhen Sunxing Light Alloys Materials Co., Ltd. (China) — The invention provides a potassium cryolite for aluminum electrolysis industry, which has a molecular formula: mKF.AlF3, wherein m is from 1 to 1.5. The low-molecular-ratio potassium cryolite (mKF.AlF3, and m is from 1 to 1.5) provided by the invention is used for aluminum electrolysis industry, and can improve the dissolvability of aluminum oxide, thus reducing the temperature of electrolysis and the consumption of power, raising the efficiency of electrolysis and lowering the comprehensive production cost. The potassium cryolite prepared using the current industrial synthesis methods generally has a molecular ratio m between 2.0 and 3.0, so it is difficult to prepare low-molecular-ratio potassium cryolite, which is pure and extremely low in water content and has a molecular ratio m between 1.0 and 1.5 as in the aforesaid invention.
US10151038 — ELECTROLYTIC DEVICE AND ANODE ASSEMBLY INTENDED FOR THE PRODUCTION OF ALUMINUM, ELECTROLYTIC CELL AND APPARATUS COMPRISING SUCH A DEVICE — Rio Tinto Alcan International Limited (Canada) — An electrolysis device comprising a pot shell (3) and an inner lining (5) defining an opening (16) through which an anode block (15) suspended from an anode support (13, 17) forming an anode assembly (12) moves vertically by means of an anode receiver (25), said anode receiver being placed outside a space defined by the top of said anode block (15), said anode receiver comprising an anode contact surface (27) working in conjunction with the anode support (13, 17) to establish therewith electrical contact and mechanical contact to moving the anode assembly (12) vertically. An anode assembly (12). An electrolytic cell and an electrolysis installation comprising such an anode assembly. The present invention relates to an electrolysis device associated with an electrolytic pot to facilitate anode replacement maneuvers and promote accessibility for handling tools and work in the electrolytic pot. The invention also aims to allow anode replacement maneuvers to be performed without stopping the production of aluminum in the pot. The invention also aims to limit wear of, and damage to the anode conductors during anode replacement maneuvers.
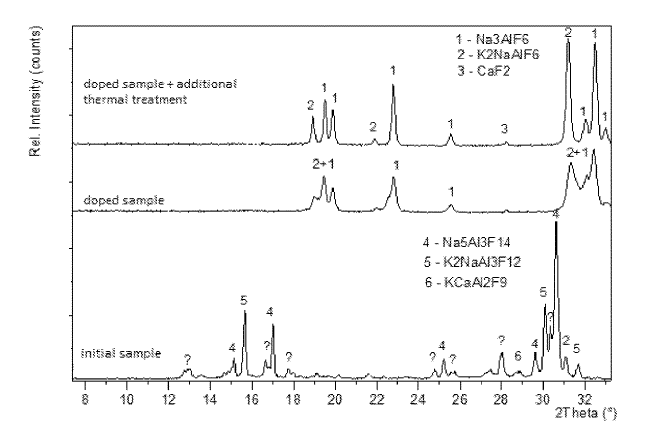
US10073049 — METHOD FOR DETERMINING THE COMPOSITION AND CRYOLITE RATIO OF SOLID SAMPLES OF POTASSIUM-CONTAINING ELECTROLYTE IN ALUMINUM PRODUCTION BY XRD — United Company Rusal Engineering and Technology Centre LLC (Russia) — This invention relates to producing aluminum by electrolysis of a melt and can be used in the process control of an electrolyte composition by quantitative X-ray phase analysis (XRD) of potassium-containing electrolyte with calcium or calcium and magnesium additives. A quantitative XRD method is employed for analyzing doped samples of crystallized bath samples taken from baths. A weighted ground bath sample is mixed with a weighted quantity of sodium fluoride at a ratio, for example, 1:2 by weight. The weighted quantities are mixed and placed in a furnace (650-750°C. for 20-40 minutes) to dissolve sodium fluoride in the sample and recrystallize the sample with the desired phase composition. The doped sample is placed in a furnace (420-450°C) and held for 15-30 minutes. The doped sample is removed from the furnace and allowed to air cool. The phase composition of the doped sample is analyzed by any quantitative X-ray phase method.