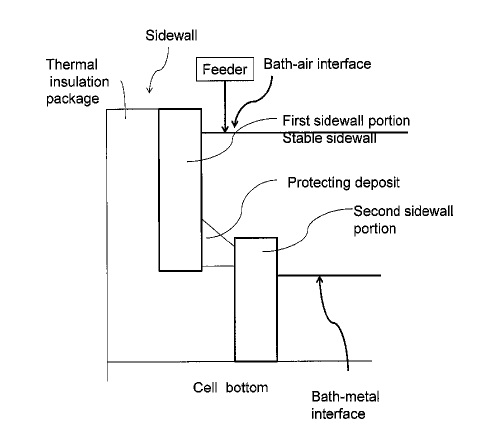
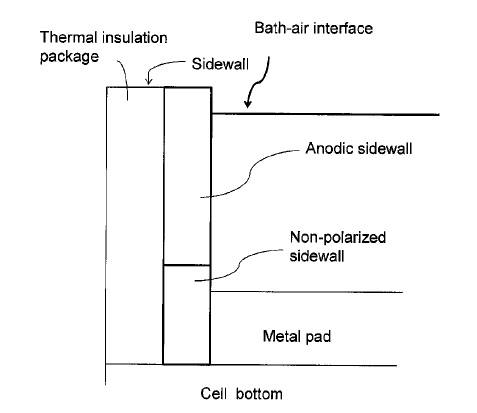
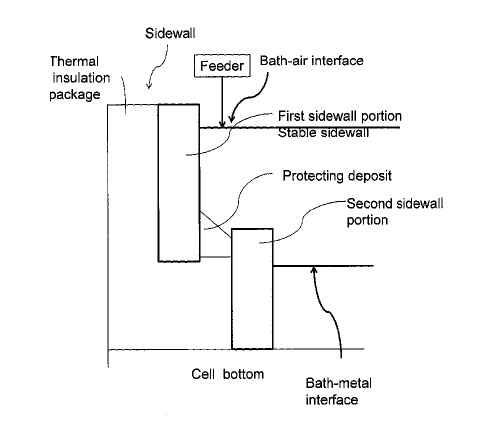
US10151039 — SYSTEMS AND METHODS OF PROTECTING ELECTROLYSIS CELL SIDEWALLS — Alcoa USA Corp. (USA) — A system is provided including an electrolysis cell configured to retain a molten electrolyte bath, the bath including at least one bath component, the electrolysis cell including: a bottom, and a sidewall consisting essentially of the at least one bath component; and a feed material including the least one bath component to the molten electrolyte bath such that the at least one bath component is within 30% of saturation, wherein, via the feed material, the sidewall is stable in the molten electrolyte bath.
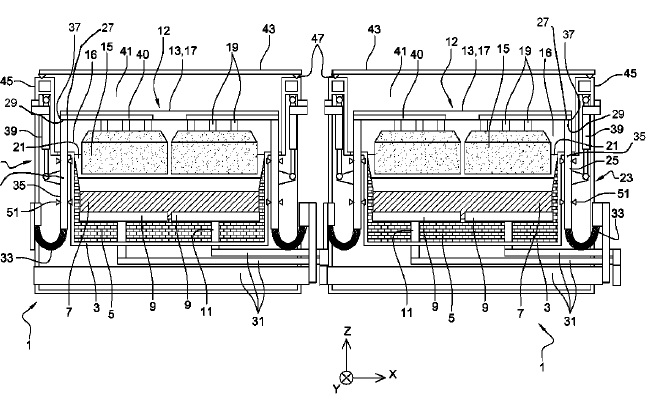
US10151038 — ELECTROLYTIC DEVICE AND ANODE ASSEMBLY INTENDED FOR THE PRODUCTION OF ALUMINUM, ELECTROLYTIC CELL AND APPARATUS COMPRISING SUCH A DEVICE — Rio Tinto Alcan International Limited (Canada) — An electrolysis device comprising a pot shell (3) and an inner lining (5) defining an opening (16) through which an anode block (15) suspended from an anode support (13, 17) forming an anode assembly (12) moves vertically by means of an anode receiver (25), said anode receiver being placed outside a space defined by the top of said anode block (15), said anode receiver comprising an anode contact surface (27) working in conjunction with the anode support (13, 17) to establish therewith electrical contact and mechanical contact to moving the anode assembly (12) vertically. An anode assembly (12). An electrolytic cell and an electrolysis installation comprising such an anode assembly.
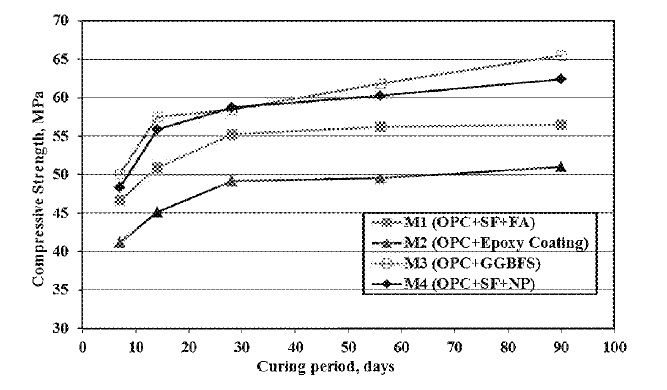
US10150703 — CEMENTITIOUS BLEND AND CONCRETE MIX COMPOSITIONS RESISTANT TO HIGH TEMPERATURES AND ALKALINE CONDITIONS — King Fahd University of Petroleum and Minerals (Saudi Arabia) — A cementitious blend composition and a concrete mix composition preferable for making concrete resistant to high temperatures and alkaline conditions, particularly for making durable concrete for constructing an alumina digester tank in an aluminum smelter. The cementitious blend composition includes at least one hydraulic cement, silica fume (SF), and natural pozzolan (NP), wherein a weight percent ratio of at least one hydraulic cement: SF:NP in the cementitious blend composition lies in the range of (24-63): (5-44): (32-40) with the sum of the weight percentages of the at least one hydraulic cement, the SF, and the NP not exceeding 100%. The concrete mix composition comprises water and the cementitious blend composition, wherein a weight ratio of the water the cementitious blend composition is 0.2-0.5, and wherein the concrete mix composition has a content of the cementitious blend composition of 400-550 kg/m3.
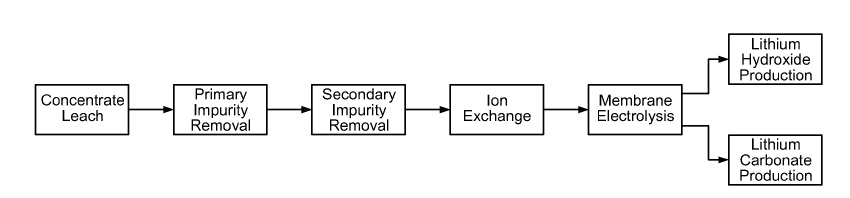
US10144990 — PROCESSES AND SYSTEMS FOR PREPARING LITHIUM CARBONATE — Nemaska Lithium Inc. (Canada) — There are provided processes comprising submitting an aqueous composition comprising lithium sulphate and/or bisulfate to an electrolysis or an electrodialysis for converting at least a portion of said sulphate into lithium hydroxide. During electrolysis or electrodialysis, the aqueous composition is at least substantially maintained at a pH having a value of about 1 to about 4; and converting said lithium hydroxide into lithium carbonate, which can be used as an additive in aluminum elecrolysis. Alternatively, lithium sulfate and/or lithium bisulfate can be submitted to a first electromembrane process that comprises a two-compartment membrane process for conversion of lithium sulfate and/or lithium bisulfate to lithium hydroxide, and obtaining a first lithium-reduced aqueous stream and a first lithium hydroxide-enriched aqueous stream; and submitting said first lithium-reduced aqueous stream to a second electromembrane process comprising a three-compartment membrane process to prepare at least a further portion of lithium hydroxide and obtaining a second lithium-reduced aqueous stream and a second lithium-hydroxide enriched aqueous stream.
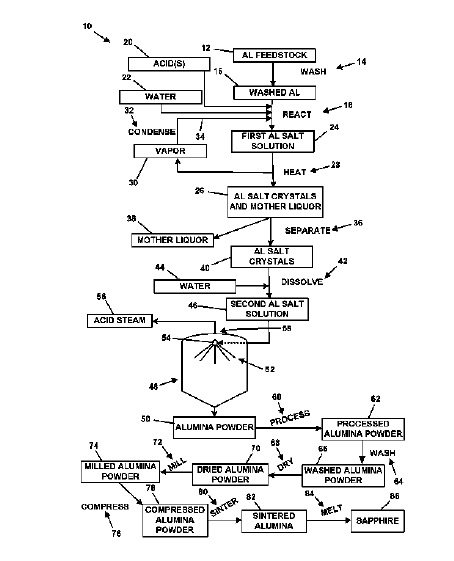
US10081553 — PROCESS FOR MAKING HIGH-PURITY ALUMINUM OXIDE — Polar Sapphire, Ltd. (Canada) — A method comprising (a) reacting aluminum metal with an acid in the presence of water to provide a first aluminum salt solution comprising an aluminum salt in water, wherein the aluminum salt comprises a reaction product of the acid and the aluminum metal, (b) heating the first aluminum salt solution to provide a mother liquor and solid aluminum salt, (c) optionally, separating the solid aluminum salt from the mother liquor, (d) optionally, dissolving at least a portion of the separated solid aluminum salt with water to provide a second aluminum salt solution, (e) spray roasting the first, or second (if produced), aluminum salt solution to provide an aluminum oxide powder, and (f) washing the aluminum oxide powder, wherein the washed aluminum oxide powder comprises less than about 30 ppmw total metallic and alkyl impurities.
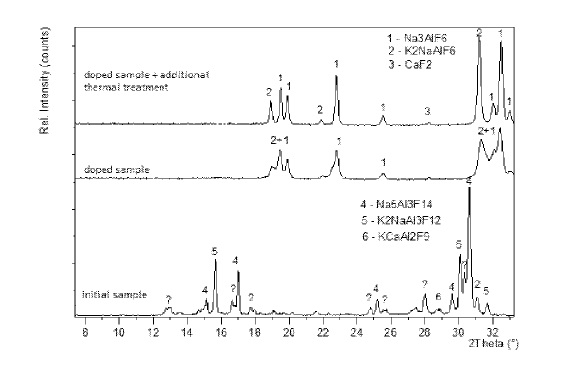
US10073049 — METHOD FOR DETERMINING THE COMPOSITION AND CRYOLITE RATIO OF SOLID SAMPLES OF POTASSIUM-CONTAINING ELECTROLYTE IN ALUMINUM PRODUCTION BY XRD — United Company RUSAL Engineering and Technology Centre, LLC (Russia) — This invention relates to producing aluminum by electrolysis of a melt and can be used in the process control of an electrolyte composition by quantitative X-ray phase analysis (XRD) of potassium-containing electrolyte with calcium or calcium and magnesium additives. A quantitative XRD method is employed for analyzing doped samples of crystallized bath samples taken from baths. A weighted ground bath sample is mixed with a weighted quantity of sodium fluoride at a ratio, for example, 1:2 by weight. The weighted quantities are mixed and placed in a furnace (650-750°C for 20-40 minutes) to dissolve sodium fluoride in the sample and recrystallize the sample with the desired phase composition. The doped sample is placed in a furnace (420-450°C) and held for 15-30 minutes. The doped sample is removed from the furnace and allowed to air cool. The phase composition of the doped sample is analyzed by any quantitative X-ray phase method.
US9996074 — SYSTEM AND PREDICTIVE MODELING METHOD FOR SMELTING PROCESS CONTROL BASED ON MULTI-SOURCE INFORMATION WITH HETEROGENEOUS RELATEDNESS — International Business Machines Corporation (USA) — The present invention is a system and predictive modeling method specially designed to improve process control and energy efficiency for a smelting process used to produce pure metal from an ore containing said metal. Data is collected from various sensor sources in the smelting process to predict whether an increase or decrease is needed in controlling two variables comprising temperature and an additive that allows the reaction in the electrolytic process to proceed at a lower bath temperature. The invention provides a generalized framework to learn the complex heterogeneity embedded in the collected data, and employs a regularized non-negative matrix factorization problem, which simultaneously decomposes the instance-feature and instance-label matrices, while enforcing task relatedness, feature type consistency and label correlations on the collected data. The predictive modeling method disclosed herein effectively mines the hidden correlation among the heterogeneous data and improves the prediction accuracy.
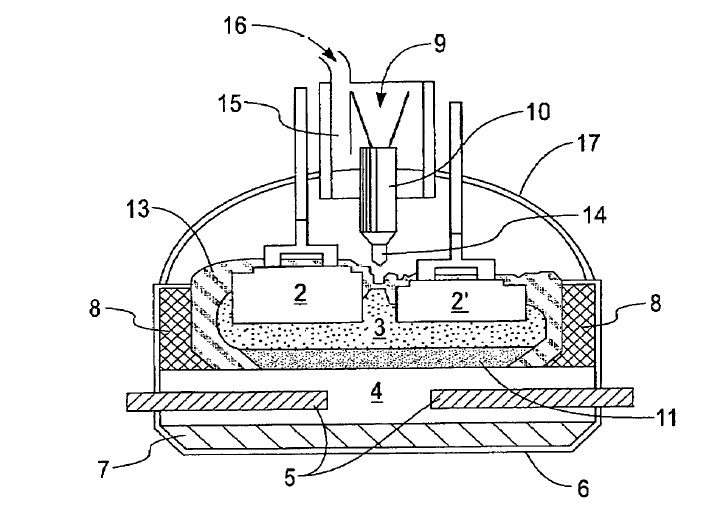
US9982355 — SYSTEMS AND METHODS FOR PREVENTING THERMITE REACTIONS IN ELECTROLYTIC CELLS — Alcoa USA Corp. (USA) — A method of monitoring an electrolytic cell including detecting information indicative of a thermite reaction, comparing the information indicative of a thermite reaction to a threshold, generating a thermite response signal according to the comparison, and reacting to the thermite response signal by adjusting the operation of the electrolytic cell.
US9957627 — SYSTEMS AND METHODS OF PROTECTING ELECTROLYSIS CELL SIDEWALLS — Alcoa USA Corp. (USA) — Broadly, the present disclosure relates to sidewall features (e.g. inner sidewall or hot face) of an electrolysis cell, which protect the sidewall from the electrolytic bath while the cell is in operation (e.g. producing metal in the electrolytic cell).
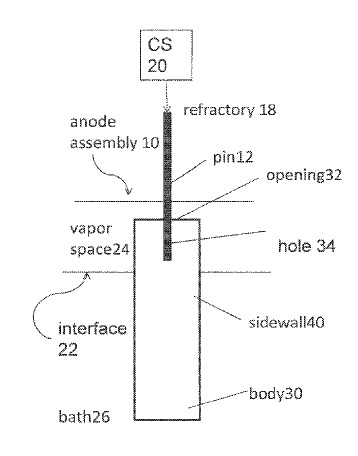
US9945041 — ANODE APPARATUS — Alcoa USA Corp. (USA) — The present disclosure related to an inert anode which is electrically connected to the electrolytic cell, such that a conductor rod is connected to the inert anode in order to supply current from a current supply to the inert anode, where the inert anode directs current into the electrolytic bath to produce nonferrous metal (where current exits the cell via a cathode).
US9932681 — ELECTROLYTIC CELL — Saint-Gobain Centre De Recherches Et D’Etudes Europeen (France) — The present disclosure relates to an electrolytic cell for the production of aluminum by reducing alumina. The cell may comprise a sidewall including at least one side block. The side block may comprise an aluminous material having an apparent porosity of less than about 10% and a composition, as a weight percentage on the basis of the aluminous material and for a total of about 00%, such that: Al2O3>about 50%, beta-alumina being less than about 20% of the weight of the aluminous material, oxides that are less reducible than alumina at 1000°C
US9902622 — METHOD FOR PREPARING ZIRCONIUM BORIDE AND SYNCHRONOUSLY PREPARING CRYOLITE — Shenzhen Sunxing Light Alloys Materials Co., Ltd. (China) — A method for preparing zirconium boride and synchronously preparing a cryolite is provided which includes the following steps: Step A: placing aluminum in a reactor, heating the reactor to 700-850oC, and adding the mixture of fluorozirconate and fluoborate; and Step B: stirring the reactants for 4-6 hours and extracting the upper molten liquid to obtain a cryolite, wherein the lower substance is zirconium boride. The disclosure has the following beneficial effects: the new zirconium boride preparation method provided herein is simple in preparation flow and the device used, short in preparation period and high in reaction efficiency, the prepared zirconium boride with many contact angles has a large specific surface area and contains a controllable amount of aluminum. With an excellent wettability to molten aluminum and a resistance to the corrosion from cryolite, zirconium boride is highly suitable for a coating on the surface of a carbon cathode. Due to their high price, borides of transition metals such as zirconium boride are difficult to be widely applied to the coating on the surface of a carbon cathode at present.
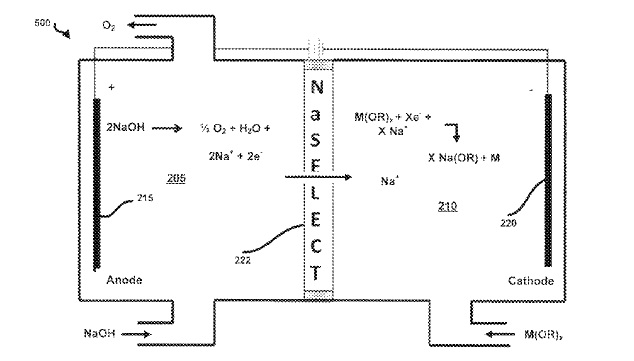
US9856569 — APPARATUS AND METHOD OF PRODUCING METAL IN A NASICON ELECTROLYTIC CELL — Field Upgrading Limited (Canada) — A process of producing metal that includes adding a quantity of an alkoxide (M(OR)x) or another metal salt to a cathode compartment of an electrolytic cell and electrolyzing the cell. This electrolyzing causes a quantity of alkali metal ions to migrate into the cathode compartment and react with the metal alkoxide, thereby producing metal and an alkali metal alkoxide. In some embodiments, the alkali metal is sodium such that the sodium ions will pass through a sodium ion selective membrane, such as a NASICON membrane, into the cathode compartment.
US9771659 — SYSTEMS AND METHODS OF PROTECTING ELECTROLYSIS CELL SIDEWALLS — Alcoa USA Corp. (USA) — A system is provided including an electrolysis cell configured to retain a molten electrolyte bath, the bath including at least one bath component, the electrolysis cell including: a bottom, and a sidewall consisting essentially of the at least one bath component; and a feeder system, configured to provide a feed material including the least one bath component to the molten electrolyte bath such that the at least one bath component is within 2% of saturation, wherein, via the feed material, the sidewall is stable in the molten electrolyte bath.
US9624134 — TITANIUM DIBORIDE GRANULES AS EROSION PROTECTION FOR CATHODES — 3M Innovative Properties Company (USA) — The invention relates to titanium diboride granules comprising aggregates of titanium diboride primary particles, wherein the titanium diboride granules have a rounded shape and are fracture-resistant. The invention further relates to a method for producing these titanium diboride granules, the use thereof for covering graphite cathodes in electrolytic cells in Al fused-salt electrolysis or for repairing holes in cathode bases of electrolytic cells and a method for repairing holes in cathode bases of electrolytic cells.
US9611151 — ELECTROLYTE SUPPLEMENT SYSTEM IN ALUMINUM ELECTROLYSIS PROCESS AND METHOD FOR PREPARING THE SAME — Shenzhen Sunxing Light Alloys Materials Co., Ltd. (China) — The disclosure provides an electrolyte supplement system in an aluminum electrolysis process, which includes low-molecular-ratio cryolite, wherein the low-molecular-ratio cryolite is selected from mKF.AlF3, nNaF.AlF3 or mixture thereof, where m=1.about.1.5 and n=1.about.1.5. When the electrolyte supplement system provided by the disclosure is applied to the aluminum electrolytic industry, electrolytic temperature can be reduced obviously in the aluminum electrolysis process without changing the existing electrolytic process; thus, power consumption is reduced, volatilization loss of fluoride is reduced and the comprehensive cost of production is reduced.
US9267214 — ALUMINUM RECOVERY PROCESS — Board of Trustees of the University of Alabama (USA) — Disclosed are processes and electrolytic cells that can be used to extract and thereby recover aluminum from aluminum-containing waste, including an aluminum dross that is suitable for disposal in a land-fill. The disclosed processes and cells use ionic liquids as an electrolyte.
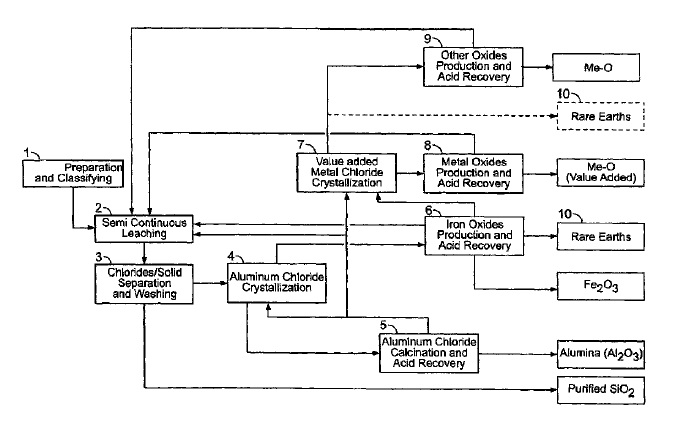
US9228248 — METHOD OF RECOVERING RARE-EARTH ELEMENTS — Nippon Light Metal Company, Ltd. (Japan) — Provided is a method of recovering rare-earth elements by which rare-earth elements can be recovered efficiently from a bauxite residue serving as a raw material and containing the rare-earth elements. Specifically provided is a method of recovering rare-earth elements from a raw material, the raw material being a bauxite residue produced as a by-product in a Bayer process, the method including: using a bauxite residue having a specific surface area of 35 m2/g or more; adding, to the raw material bauxite residue, a liquid leaching agent formed of an aqueous solution of at least one kind of mineral acid selected from sulfuric acid, hydrochloric acid, nitric acid, and sulfurous acid, thereby preparing a slurry having a liquid-solid ratio of 2 to 30 and a pH of 0.5 to 2.2; subjecting the slurry to leaching treatment of the rare-earth elements under a temperature condition of room temperature to 160°C.; subjecting the slurry after the leaching treatment to solid-liquid separation, yielding a leachate; and separating and recovering the rare-earth elements from the leachate.
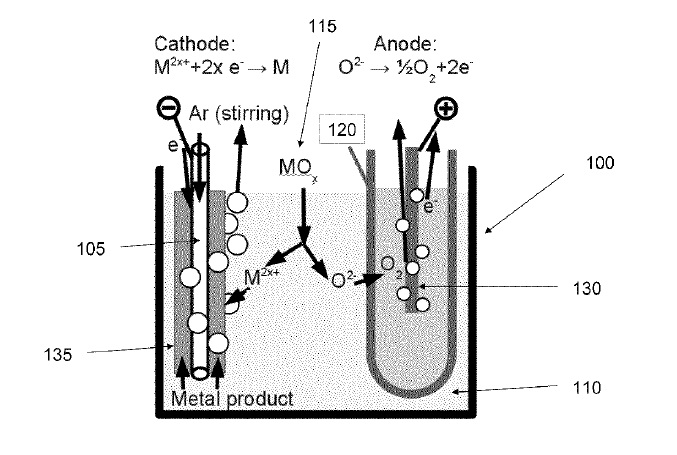
US9206516 — LIQUID ANODES AND FUELS FOR PRODUCTION OF METALS FROM THEIR OXIDES BY MOLTEN SALT ELECTROLYSIS WITH A SOLID ELECTROLYTE — Infinium, Inc. (USA) — In one aspect, the present invention is directed to liquid anodes and fuels for production of metals from their oxides. In one aspect, the invention relates apparatuses for producing a metal from a metal oxide comprising a cathode in electrical contact with an electrolyte, a liquid metal anode separated from the cathode and the electrolyte by a solid oxygen ion conducting membrane, a fuel inlet, and a power supply for establishing a potential between the cathode and the anode. In another aspect, the invention relates to methods for production of metals from their oxides comprising providing a cathode in electrical contact with a molten electrolyte, providing a liquid metal anode separated from the cathode and the molten electrolyte by a solid oxygen ion conducting membrane, providing a fuel inlet, delivering a gaseous fuel comprising hydrogen to the liquid metal anode via the fuel inlet, and establishing a potential between the cathode and the anode.
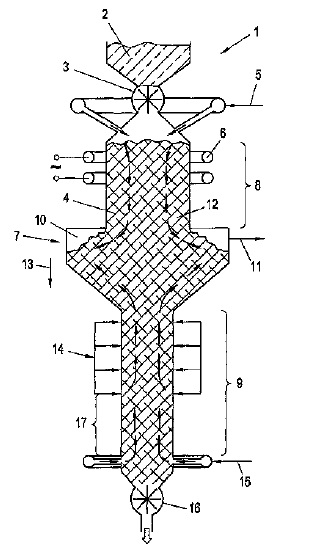
US9199109 — METHOD FOR PROCESSING USED CARBON-CONTAINING CATHODE MATERIAL — SGL Carbon SE (Germany) — In a method for processing used cathode material containing carbon, in particular used cathode troughs from aluminum production, the cathode material is put into a shaft furnace and, in order to gasify carbon, is subjected to a thermal treatment in the shaft furnace at a temperature above the ignition temperature of the carbon and above the evaporation temperature of toxic substances contained in the used cathode material. The reaction gases are conducted co-current with the carbon in a first longitudinal section of the shaft furnace and countercurrent to the carbon in a second longitudinal section of the shaft furnace. The reaction gases are drawn from a region of the shaft furnace having an enlarged cross-section between the longitudinal sections and are preferably subjected to an after-treatment.
US9181603 — PROCESSES FOR TREATING FLY ASHES — Orbite Technologies Inc. (Canada) — There are provided processes for treating fly ash. For example, the processes can comprise leaching fly ash with HCl so as to obtain a leachate comprising aluminum ions and a solid, and separating the solid from the leachate; reacting the leachate with HCl so as to obtain a liquid and a precipitate comprising the aluminum ions in the form of AlCl3, and separating the precipitate from the liquid; and heating the precipitate under conditions effective for converting AlCl3 into Al2O3 and optionally recovering gaseous HCl so-produced.
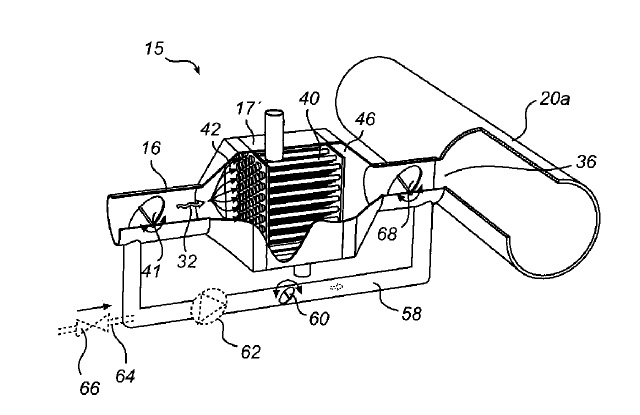
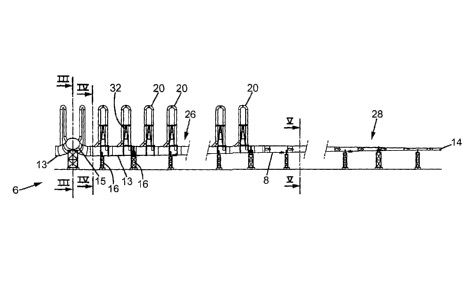
US9115487 — RAW GAS COLLECTION SYSTEM — Alstom Technology Ltd. (Switzerland) — The present invention relates to a raw gas collection system for collecting raw gas from a plurality of aluminum smelting pots. A plurality of branch ducts is adapted to channel raw gas from a respective aluminum smelting pot. A common collecting duct channels the raw gas from the plurality of branch ducts to a gas cleaning unit. The present invention also relates to a method for balancing a flow of raw gas in a raw gas collection system, and to the use of a plurality of combined heat transfer and flow resistance generating elements for balancing a plurality of branch flows of raw gas in a raw gas collection system. Thus, A raw gas collection system (15) for collecting raw gas from a plurality of aluminum smelting pots (4) is equipped with a plurality of branch ducts (16, 16a-d). Each branch duct (16, 16a-d) is arranged to channel a respective branch flow (32, 32a-b) of raw gas from an aluminum smelting pot (4) to a collecting duct (20a), which is common to and shared by branch ducts (16, 16a-d). Several of the branch ducts (16, 16a-d) are equipped with a combined heat transfer and flow resistance generating element (17) to remove heat from the respective branch flow (32, 32a-b) of raw gas and to balance the flow of raw gas in the raw gas collecting system (15). The combined heat transfer and flow resistance generating elements (17) reduce the need for adjusting the respective branch duct (16, 16a-d) flow volumes using dampers, thereby reducing the power required to transport the raw gas through the system.
US9068249 — RARE EARTH ELEMENT RECOVERY METHOD — Nippon Light Metal Company, Ltd. (Japan) — Provided is a method of recovering rare-earth elements, including causing rare-earth elements particularly including Nd and Dy to leach efficiently from a raw material for leaching which contains the rare-earth elements, and separating and recovering the rare-earth elements. The method of recovering rare-earth elements includes: a leaching step including performing leaching treatment of rare-earth elements in which an acidic slurry of a raw material for leaching which contains the rare-earth elements is held under a predetermined condition, and then subjecting the slurry obtained after the leaching treatment to solid-liquid separation, yielding a leachate containing the rare-earth elements; and a separation step of separating and recovering the rare-earth elements from the yielded leachate, in which: the raw material for leaching contains Ca as CaO at a ratio of 4 to 15 mass % and Ti as TiO2 at a ratio of 2 to 13 mass % in a solid component (S); an acid aqueous solution is an acid aqueous solution of hydrochloric acid and/or nitric acid; and the leaching treatment performed in the leaching step is digestion or maceration which is performed under the heating and pressurizing conditions of a temperature of 160 to 300°C. and a pressure of 0.65 to 10 MPa, and the rare-earth elements are caused to leach together with Ca in the leaching step.
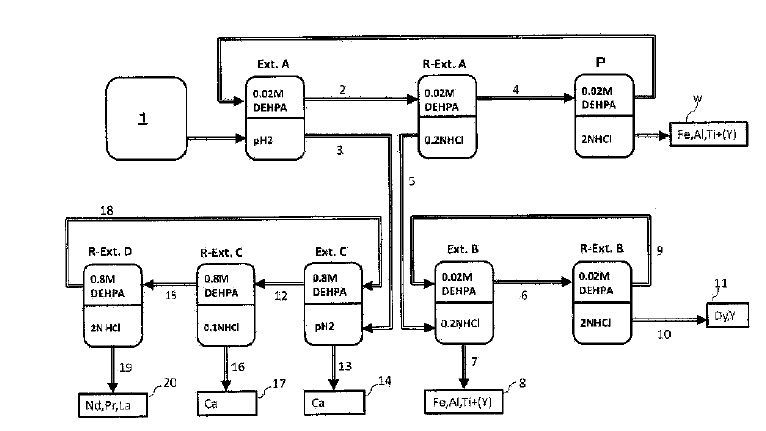
US9023301 — PROCESSES FOR TREATING RED MUD — Orbite Aluminae Inc. (Canada) — The present disclosure relates to improvements in the field of processes for treating industrial waste materials. For example, it relates to processes for treating red mud. For example, these processes can be effective for extracting various materials from red mud such as alumina and various metals and oxides thereof, silica, rare earth elements, rare metals etc. There are provided processes for treating red mud. For example, the processes can comprise leaching red mud with HCl to obtain a leachate comprising ions of a first metal (for example aluminum) and a solid, then separating said solid from said leachate. Several other metals can be extracted from the leachate (Fe, Ni, Co, Mg, rare earth elements, rare metals, etc.). Various other components can be extracted from solid such as TiO2, SiO2, etc.
CA2977410 — ANODE ASSEMBLY AND METHOD FOR MANUFACTURING ANODE ASSEMBLY — Hatch Ltd. (Canada) — An aluminum electrolysis cell anode assembly has an anode, a stub and a thimble. The stub is inserted into a stub hole of the anode. The thimble is formed between the stub and the anode within the stub hole. A lateral wall of the stub has a discontinuity, which may be a recess, a protrusion or a combination thereof. An inner surface of the thimble engages the discontinuity of the stub. The engagement restricts axial movement of the thimble relative to the stub. Under thermal expansion of the anode assembly, the base of the stub is urged towards the stub hole bottom and stub-to-anode resistance is improved. This further improves efficiency of an aluminum electrolysis process.
CA2910233 — CATHODE BLOCK HAVING A SLOT WITH VARYING DEPTH AND A SECURING SYSTEM — SGL CFL CE GmbH (Germany) — A cathode block for an aluminum electrolysis cell based on carbon and/or graphite has at least one slot for receiving at least one bus bar, said slot extending in the longitudinal direction of the cathode block, wherein at least one of the at least one slot has a depth that varies when viewed over the length of the cathode block, and wherein at least one recess which extends horizontally in the longitudinal direction of the cathode block is provided in the cathode block wall bordering the at least one slot. According to another embodiment, a cathode block for an aluminum electrolysis cell based on carbon and/or graphite has at least one slot for receiving at least one bus bar, said slot extending in the longitudinal direction of the cathode block, wherein at least one of the at least one slot has a depth that varies when viewed over the length of the cathode block, and wherein this slot is bordered by a wall, at least one projection that projects into the slot being provided on said wall.
CA2910088 — CATHODE BLOCK HAVING A SLOT WITH A VARYING DEPTH AND A FILLED INTERMEDIATE SPACE — SGL CFL CE GmbH (Germany) — A cathode block for an aluminum electrolysis cell based on carbon and/or graphite, wherein the cathode block has at least one slot which extends in the longitudinal direction of the cathode block, wherein at least one of the at least one slots has a depth which varies, as seen over the length of the cathode block, and at least one busbar is provided in the at least one slot, wherein the intermediate space between the at least one busbar and the wall which bounds the at least one slot with a varying depth is at least partially filled with steel.
CA2891214 — ALUMINUM ELECTROLYSIS CELL CATHODE SHUNT DESIGN — Obshchestvo S Ogranichennoy Otvetstvennost’yu “Obedinennaya Kompaniya Rusal Inzhenerno-Tekhnologicheskiy Tsentr” (Russia) — The invention relates to non-ferrous metallurgy, in particular, electrowinning of aluminum from cryolite-alumina melts, and can be used in the shunt design of a cathode assembly. In an aluminum electrolysis cell, cathode vertical metal shunts, which conduct electrical current from the aluminum melt to the cathode busbar, are designed such that their top part is melted aluminum, and the bottom part is solid aluminum. Shunts are located in conduits made in a hearth slab lining which has a widening in the middle part which is wider than both parts of the shunts. The widening in the shunt conduit can be filled with a composite material, i.e. titanium diboride-carbon. The shunts can be designed as a tube, and the widening in the conduit and the space inside the tube can be filled with the composite material titanium diboride-carbon. The application of the proposed technical solution makes it possible to significantly increase the electrical efficiency due to the absence of contact assemblies which contain dissimilar materials in the cathode shunt, due to reduced current loss, and due to achieving a guaranteed effective current distribution and an effective current shunting.
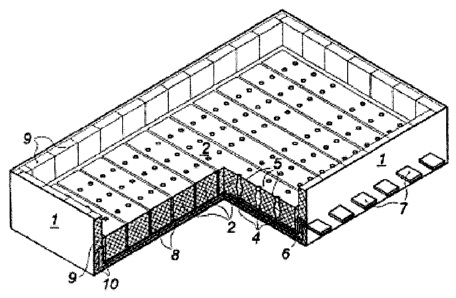
CA2877591 — ELECTROLYTIC CELL FOR ALUMINUM ELECTROLYSIS AND ELECTROLYSIS PROCESS USING ELECTROLYTIC CELL — Inner Mongolia United Industrial Co., Ltd. (China) — An electrolysis tank used for aluminum electrolysis, comprising a tank body, an anode and a cathode arranged within the tank body, also an electrolyte accommodated within the tank body, where at least a part of the anode is submerged in the electrolyte. The anode is arranged above the tank body. The cathode is arranged at the tank bottom and is covered by a certain amount of liquid aluminum. The electrolyte is provided between the anode and the cathode. The electrolyte covers the liquid aluminum. The tank body has arranged on an inner sidewall thereof an insulation layer for use in separating oxygen or the electrolyte from a carbon block. The tank is characterized in that: the constituents of the anode comprise Fe, Cu, Ni, and Sn, where Fe and Cu are the main constituents; the electrolyte consists of 30 to 38 wt% of NaF, 49 to 60 wt% of AlF3, 1 to 5 wt% of LiF, 1 to 6 wt% of KF, and 3 to 6 wt% of Al2O3, where the molar ratio of NaF to AlF3 is between 1.0 and 1.52. The electrolysis tank is applicable in industrialized production of electrolyzed aluminum.
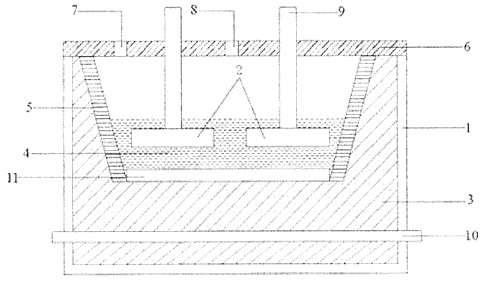
CA2876345 — ELECTROLYTE FOR ALUMINUM ELECTROLYSIS AND ELECTROLYSIS PROCESS USING ELECTROLYTE — Inner Mongolia United Industrial Co., Ltd. (China) — The present invention relates to an electrolyte used for aluminum electrolysis and an electrolysis process using the electrolyte. The electrolyte of the present invention employs a pure fluoride salt system and consists of the following mass percent of constituents: 20% to 29.9% of NaF, 60.1% to 66% of A1F3, 3% to 10% of LiF, 4% to 13.9% of KF, and 3% to 6% of Al2O3, where the molar ratio of NaF to AlF3 is between 0.6 and 0.995; or consists of the following mass percent of constituents: 30% to 38% of NaF, 49% to 60% of AlF3, 1% to 5% of LiF, 1% to 6% of KF, and 3% to 6% of A12O3, where the molar ratio of NaF to AlF3 is between 1.0 and 1.52. The electrolyte provided in the present invention is provided with a reduced primary crystallization temperature and improved solubility of aluminum oxide, while the electrolyte is provided with an increased electric conductivity. The electrolyte of the present invention provides improved effects of reduced energy consumption, increased current efficiency, and improved working environment during the electrolysis process.
CA2876336 — INERT ALLOY ANODE FOR ALUMINUM ELECTROLYSIS AND PREPARING METHOD THEREOF — Inner Mongolia United Industrial Co., Ltd. (China) — An inert alloy anode used for aluminum electrolysis. The anode has Fe and Cu as the main constituents and comprises Sn. The addition of the Sn metal is conducive to the formation of a layer of oxidized film having a great antioxidant activity and structural stability on the surface of the inert alloy anode and is conducive to an increase in the corrosion resistance of the anode. On this basis, the constituents of the inert alloy anode also comprise Ni, Al, and Y. The addition of the Al metal prevents the main metal constituents from being oxidized, the addition of the Y metal controls the alloy to provide a required polymorph in a preparation process, thus achieving the goal of anti-oxidation. The inert alloy anode having Fe and Cu as the main constituents has a low over-voltage, high electric conductivity and reduced costs and is applicable to the aluminum electrolysis industry.
CA2872922 — DIRECT-CURRENT SHUNT PREHEATING START METHOD FOR INERT ELECTRODE ELECTROLYSIS CELL — Aluminum Corporation of China Limited (China) — Disclosed is a direct-current shunt preheating start method for an inert electrode aluminum electrolysis cell, comprising: (1) forming multiple direct-current shunt element groups by using conductors with preset resistance values and geometric sizes; (2) laying in a hearth of the electrolysis cell electrical heating element groups of the same number as/a different number from electrode groups; (3) drying the hearth, smelting electrolyte, and establishing a thermal balance and a hearth model by using the electrical heating element groups and according to a set heating curve or set steps; (4) changing the number of groups of/a series or parallel state of the direct-current shunt elements; and (5) gradually replacing inert electrodes, and gradually adjusting the number of the groups of/the series or parallel state of the shunt elements. By means of the present invention, the inert electrode aluminum electrolysis cell can be well preheated and the thermal balance can be established; in the inert electrode replacement process, stability of the cell voltage can further be ensured, so that the current passing through the inert electrodes in the cell is uniform; and series current is not affected by start of a single electrolysis cell, so that non-disturbance start is implemented.
CA2834290 — METHOD FOR UNIFORMING DISTRIBUTION OF CURRENT IN ALUMINUM LIQUID IN AN ALUMINUM ELECTROLYTIC TANK — China Aluminum International Engineering Corporation Limited (China) — Disclosed is a method for uniforming the distribution of current in an aluminum electrolysis cell. At least one cathode steel bar is fixedly tied or casted at the lower part of a cathode carbon block, and the cathode steel bar is cut into several sections located at different places along the length direction by a separating seam. The said sections upon the separating seams of the cathode steel bar except those between the separating seams are totally connected with the cathode carbon block by conductive bodies, and the sections between and below the separating seams of the cathode carbon steel bar are insulated from the cathode carbon block by insulators. Insulating materials for separating seam are filled in the separating seam, so that the sections upon and below the separating seams of the cathode steel bar are insulated from each other, and one end of the cathode steel bar penetrates out of the electrolytic cell from its sidepiece. The cathode current of the aluminum electrolysis cell is distributed more uniformly, and the horizontal current in the aluminum liquid is reduced, then the stability of the electrolytic cell is improved, and the aluminum electrolysis cell can be efficiently circulated with a low polar distance, thus the energy consumption for per ton of aluminum is reduced effectively, and the energy-saving effect is achieved, meanwhile the service life of the cathode is also prolonged.
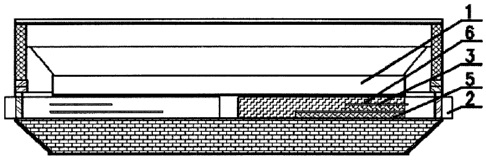
CA2792415 — CATHODE STRUCTURE, ALUMINUM ELECTROLYSIS CELL, AND METHOD FOR LOWERING HORIZONTAL CURRENT IN ALUMINUM LIQUID — China Aluminum International Engineering Corporation Limited (China) — A cathode structure of an aluminum electrolytic cell, wherein the section of the cathode steel bar adjacent to the end portion of the cathode carbon block in its lengthwise direction is divided from top to bottom by partition gap(s) into at least two portions including an upper portion in a height direction of the cathode steel bar, the section of the cathode steel bar which is in a middle portion of the cathode carbon block and not divided is connected to the cathode carbon block by using a conductor, the upper portion of the cathode steel bar adjacent to the end portion of the cathode carbon block is connected to the cathode carbon block by using the conductor, the other portions below the upper portion are insulated from the cathode carbon block by using an insulator, and the partition gap is filled with a partition gap insulating material. An aluminum electrolytic cell including the above-mentioned cathode structure and a process for reducing horizontal electric current in the liquid aluminum in the aluminum electrolytic cell are also disclosed. The present invention substantially reduces the horizontal electric current in the liquid aluminum, makes the cathode electric current more uniformly distributed, improves the stability of the electrolytic cell, prolongs the service lifetime of the electrolytic cell, effectively reduces energy consumption of producing aluminum per ton and exhibits a remarkable energy-saving effect.
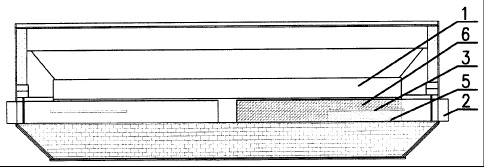
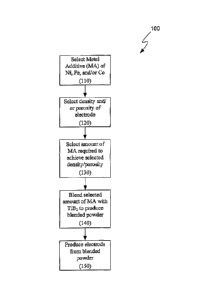
CA2768992 — COMPOSITION FOR MAKING WETTABLE CATHODE IN ALUMINUM SMELTING — Alcoa USA Corp. (USA) — Compositions for making wettable cathodes to be used in aluminum electrolysis cells are disclosed. The compositions generally include titanium diboride (TiB2) and metal additives. The amount of selected metal additives may result in production of electrodes having a tailored density and/or porosity. The electrodes may be durable and used in aluminum electrolysis cells.
CA2768648 — DEVICE FOR COLLECTING GASES EMITTED BY ALUMINUM ELECTROLYSIS TANKS AND ASSOCIATED ALUMINUM PRODUCTION SYSTEM — Solios Environnement (France) — The invention relates to a collection device (6) capable of collecting the gases emitted by multiple aluminum electrolysis tanks, characterized in that it comprises at least one bypass loop (20) for a gas flow, connected, at each of the ends thereof, to the collection device (6), said bypass loop (20) being capable of passing through a medium, the temperature of which is lower than the temperature of the gas.
CA2737182 — NOVEL COMBINED GRAPHITIZED IRREGULAR CATHODE FOR ALUMINUM AND GRAPHITIZED CATHODE STOP BLOCK THEREOF — Guangxi Qiangqiang Carbon Co. Ltd. (China) — A novel combined graphitized irregular cathode for aluminum comprises body blocks and graphitized cathode stop blocks; the graphitized cathode stop blocks are prepared from raw materials including: calcined petroleum coke, electrically calcined anthracite, coal pitch, TiB2 alloy additive and SiC additive. According to the invention, the graphitized cathode stop blocks are inlaid at a junction seam of two cathode body blocks in a manner of bridging over the cathode, thus electrode distance of aluminum electrolysis is shortened, cell voltage is lowered by about 0.35 volts to 0.5 volts; the energy per ton aluminum can be saved not less than 1000KWh to achieve prominent effect of energy-saving and consumption-reduction. The invention can avoid grooving in the middle of the body blocks and reduce the effective thickness of body blocks to impact on life-span, while implementing reinforcement of local structure on the junction seam of body blocks to prolong life-span of electrolytic cell.
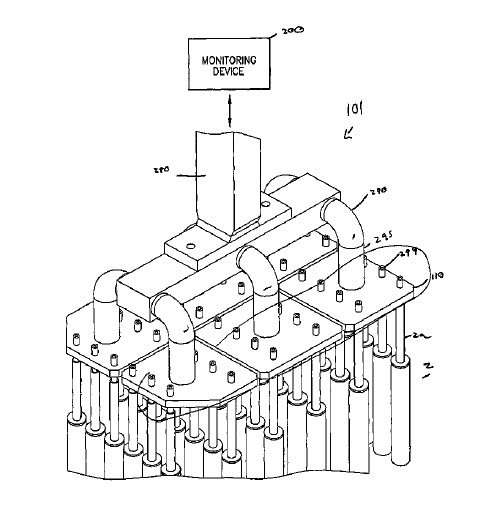
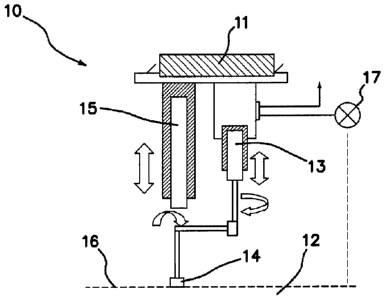
CA2755221 — SYSTEM, METHOD AND APPARATUS FOR MEASURING ELECTROLYSIS CELL OPERATING CONDITIONS AND COMMUNICATING THE SAME — Alcoa USA Corp. (USA) — System, method and apparatus for measuring electrolysis cell operating conditions and communicating the same are disclosed. The system includes a selectively positionable member coupled to an analytical apparatus, wherein the selectively positionable member is configured to move the analytical apparatus into and out of physical communication with a bath. The system may also include a crust breaker for breaking the surface of a bath and an electronic device for measuring bath level.
CA2744600 — SYSTEMS AND METHODS FOR INSPECTING ANODES AND SMELTING MANAGEMENT RELATING TO THE SAME — Alcoa USA Corp. (USA) — Systems and methods for inspecting anodes, and smelting management based thereon are provided. In one’ embodiment, a system includes an imaging device configured to obtain images of at least one anode assembly, an image processor configured to producing imaging data based on the images, and a data analyzer configured to produce anode characteristic data based on the imaging data. In one embodiment, a method includes the steps of obtaining at least one image of at least a portion of an anode assembly, producing imaging data based on the at least one image, and deriving anode characteristic data based, at least in part, on the imaging data. The anode characteristic data is further correlated to electrolysis cell data of the smelting facility.
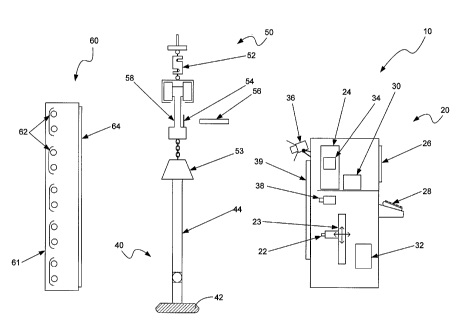
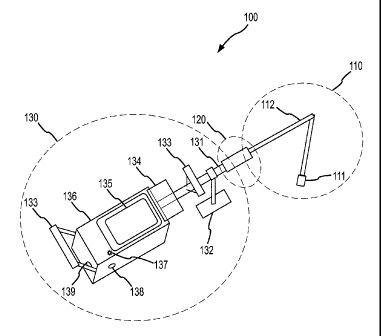
CA2656092 — SYSTEMS AND METHODS USEFUL IN CONTROLLING OPERATIONS OF METAL ELECTROLYSIS CELLS — Alcoa USA Corp. (USA) — An analysis system (100) for determining one or more operating conditions of a metal electrolysis cell and for facilitating a response is provided. The analysis system comprises a bath probe (111) electrically interconnected to a portable computing device (135). The bath probe generates signals associated with temperature measurements produced from thermal communication with an electrolysis cell bath. The portable computing device may receive these signals and generate data based thereon. The data may be transformed into operating condition information relating to the electrolysis cell.