By Aaron Palumbo, Big Blue Technologies.
Magnesium metal is trending, again. The original boom cycle of the 1930s and ’40s was sparked by the second world war, resulting in the array of foundational production technologies to which today’s processes owe a great deal. The 1980s and ’90s saw more advancement and rapid expansion of end-uses with the advent of portable electronics. With magnesium prices reaching all-time highs in 2021, major supply chain disruptions, and sensitivity to embedded environmental impacts, a new cycle has emerged. Now that magnesium is designated as a critical material by the U.S. Department of Energy and the EU Critical Raw Materials Alliance, the Western world has decried the geographic imbalance in global supply. Governments are poised to buoy many of these efforts with some participation from climate tech funds and industry leaders.
Since its original commercial production in the early 1900s, magnesium’s use has always trended upward at a modest pace. Its application as the lightest structural metal and pervasiveness in a variety of related industries underpins its importance to the greater manufacturing infrastructure. The modern example is its use for light-weighting battery electric vehicles, whose gross weight far surpasses their internal combustion counterparts. Less weight means greater range and/or smaller battery packs, reducing dependency on foreign and conflicted battery materials, such as cobalt.
However, magnesium metal production is difficult and often dangerous. Many previous projects failed because they lacked emphasis on the core smelting technology. Perhaps this is why the dominant technology is such a brute force technique. The Chinese version of the Pidgeon process, a silicothermic approach, is marred by its high labor and energy intensity. Through intense subsidization, rather than technological sophistication, the global primary production landscape has exacerbated many of the supply chain pain points for customers.
The traditional approach to bring a new metals production process to market is to acquire a land or sea asset and then select or commission a smelting technology. This business model doesn’t work for magnesium. As the eighth most abundant element in the earth’s crust, it’s well known that magnesium is not resource constrained and is “virtually inexhaustible.”1 Exploration and processing companies are looking to exploit the inferred value of their deposits or byproduct streams, only to find there are no competitive smelting technologies that will make money. With no off-the-shelf options, these entities come to find that every facility is unique, even those that deploy the same technology principles. Even silicothermic reduction means something vastly different to RIMA Industrial in Brazil than it does to Chinese Pidgeon production. Every project, then, has a unique set of risks that can only be met with a strong will and perhaps deep pockets.
There are many stakeholders from the previous and current cycles who wish to see this “minor metal” flourish into a material of significant socio-economic importance nearing or even reaching par with its light metal sister aluminum. For this to happen, technology needs to drive a price drop, while maintaining attractive margins. New suppliers also need to be reliable and stable. Restoring balance and reshoring production can only be achieved by addressing the challenging nature of magnesium, which begins with the core smelting unit operations. If the lessons of history are to be gleaned to avoid repeated failure, the Big Blue Technologies (BBT) team has placed emphasis on the smelting aspects that increase the odds of success. In doing so, the team can affirm the answer to the perpetual question: will this time be the time magnesium matures?
Foray into Aluminothermic Reduction

BBT originated from the University of Colorado at Boulder. The founders have been evaluating and innovating various thermal or pyro-metallurgical process schemes for ten years. From fundamental research to piloting, the team has looked at nearly all feasible feed material and process configurations for magnesium metal production. This technical exploration landed the team on aluminothermic reduction (ATR). With emphasis on safety and scalability, the team currently operates its ATR pilot facility outside of Cheyenne, WY (Figure 1). While validating key operational techniques, BBT has begun the transition to a 2,000 ton per year commercial demonstration plant with industrial partners.
The aluminothermic process is conceptually straightforward: aluminum metal and magnesium ore are heated to >1,000°C, essentially distilling magnesium metal from the charge and co-producing a value-add refractory. Using molten salt cooling to tightly control the condensation conditions (Figure 2), high-quality metal can be produced continuously. With state-of-the-art automation and control, the process can be operated with no-man-on-the-floor, reducing labor and setting the standard for safety. Incorporating scrap aluminum reduces costs and embedded emissions. ATR in this configuration represents the lowest energy demand of any production technology and solves the labor intensity and environmental impact problems that generally characterize thermal processing routes.

Begin at the Source
It’s worth pointing out that any magnesium metal production pathway can use virtually any magnesium-containing source. Emley articulated these foundational chemistries in his seminal work.2 Naturally occurring magnesium chloride, such as that found in the Great Salt Lake and Dead Sea at high concentrations, can be readily converted to an oxide. Similarly, magnesite, serpentine, and any other land-based ore can be converted to chloride. This is all done every day at scale by a variety of industrial players and for various markets—metal production being just one.
For example, the carbothermic magnesium metal production plant at Permanente in California used sea water as its source in the early 1940s.3 At the same time, Dow Chemical was starting up to use seawater from the Gulf Coast in its electrolytic production plant near Freeport, TX.4 Therefore, the use of seawater is not exclusive to any particular smelting technology. The truth is even more nuanced. Both Permanente and Dow Chemical’s historic processes used lime (calcined calcium carbonate) and seawater as the primary raw materials. The lime was calcined in a high temperature process, releasing direct CO2 emissions from both the combustion of fossil fuels and from the decomposition of the ore itself.
Sound familiar? The Pidgeon process must also calcine its dolomite feed. Furthermore, Dow produced a substantial amount of dross, creating a large waste pile that was notoriously named Mag Mountain.5
The emissions impact of the ATR process is dominated by where the magnesium oxide comes from, land or sea. The difference in emissions between post-industrial or post-consumer aluminum scrap feed also contributes to the overall footprint. In practice, these are levers with stark trade-offs between cost and embedded impact. Regardless, the emissions profile of the incumbent Pidgeon process is so high, that the BBT base case shows at least a 60% reduction in emissions. To get to >95% reduction or even net-zero emissions, magnesium oxide needs to originate from a non-carbonate ore or a carbon capture solution needs to be implemented for the calcination kiln. Many are working on these technologies.
Electrolytic vs. Thermal Reduction
Thermal, as a classification category, is a bit of a misnomer. Electrolytic cells operate as high as 750°C, where thermal processes can be as low as 1,000°C. For both pathways, the energy consumed to heat the system is small compared to the heat of chemical reaction to produce metal. When electrons are donated from aluminum (for ATR), the total theoretical amount of energy required is the lowest versus electrolytic and silicothermic (Table I).
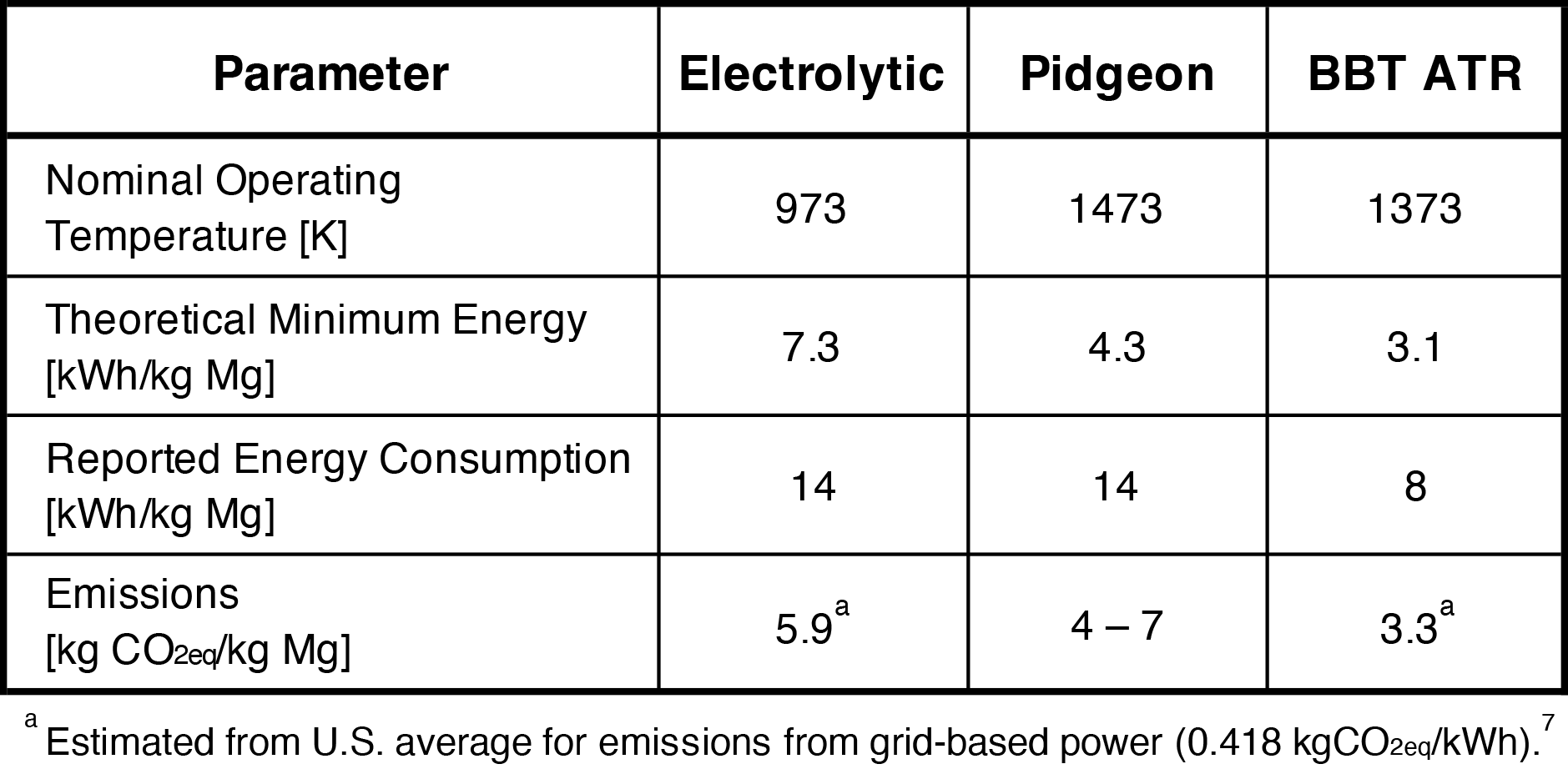
The Chinese Pidgeon process uses direct combustion of fossil fuels in the central smelting step. Thermal processes, despite their grouping with Chinese Pidgeon, can be electrified instead. The plant mentioned above, Permanente, is an example. Two electric thermal plants continue operation today in Brazil (by RIMA) and Serbia (by Mg Serbien). The high temperature furnace is usually the step that consumes the most energy. Electrification is one way the magnesium industry can future-proof its operations to avoid direct emissions and incorporate renewable power resources. Since the ATR process requires less energy than its competitors, it can achieve a low emissions impact even with grid-based power from today’s power plants.
The challenges of each technological pathway are diametrically opposed. Electrolytic plants face an upstream dehydration challenge; thermal plants need innovation on downstream condensation and recovery of the magnesium vapor. After nearly seven years of work on thermal reduction systems, the BBT team found a way to precisely control the condensation process in a fully electrified smelting operation.
Toward a Lighter Future
Magnesium’s benefits are no secret. Across a myriad of industries, magnesium is the material of choice, and would be for others if the price was lower. And that’s where the real story of magnesium lies: the state of the primary supply industry has stunted the growth of both commoditized and high value applications. The limited set of standardized magnesium alloys is speculated to be a direct consequence of the lack of participation of suppliers with downstream applications. Primary magnesium projects have been, and continue to be, fraught with failures and disappointment. Billions of dollars of losses have been written off. The history of development and commercial projects reveals explosions, loss of equipment, loss of business, loss of local and national industries, and loss of life. Now there is simply a lack of expertise. Who wants to work in this industry?
Bob Brown’s article about Australian magnesium projects still looms.8 Australia has some of the best magnesium deposits in the world and had over a dozen projects starting up in the 1990s. None of them succeeded in full-scale commercialization despite tens of millions of dollars of private and public investment.
The Pidgeon process swelled the industry up to 20 kg of Mg at a time in the late 1990s, now running millions of batches per year. The market share of magnesium from electrolytic reversed on a dime (literally about ten years), from over 70% to less than 20% today. Combined with some spectacular failures, corporate losses, and unnerving capital intensities, BBT believes the future of primary supply is held by whomever can balance cost, sustainability, and safety—a feature that only select thermal routes seem to possess.
Scaling a viable magnesium metal production concept and producing metal cost-competitively is the only way to truly support customers and enable new end-uses. On-time deliveries at consistent qualities are customers’ main requests. To conclude, consider Emley’s dedication inscription (with some updating), “To those people, in all countries, who have striven to build the magnesium industry; and to their partners who have supported their efforts with patience and understanding.”
References
- Merrill, A.M., “Magnesium Compounds,” Mineral Commodity Summaries, USGS.gov, January 2023.
- Emley, Edward F., Principles of Magnesium Technology, Pergamon Press, 1966.
- Elkins, D.A., Placek, P.L., and K.C. Dean, “An economic and technical evaluation of magnesium production methods (in three parts): 2. Carbothermic,” U.S. Department of the Interior, Bureau of Mines, 1967.
- Ball, C.J.P., “The History of Magnesium,” The Journal of the Institute of Metals, February 1956.
- Brandt, E.N., We Called it MAG-nificent: Dow Chemical and Magnesium 1916 – 1998, Michigan State University Press, 2013.
- Ehrenberger, S. “Life Cycle Assessment of Magnesium Components in Vehicle Construction,” German Aerospace Centre e.V., 2013.
- “How much of U.S. carbon dioxide emissions are associated with electricity generation?” U.S. Energy Information Administration, www.eia.gov/tools/faqs/faq.php?id=77&t=11, site visited August 11, 2023.
- Brown, R., “Magnesium Summary 2011,” Australian Journal of Mining, August 2011.
Editor’s Note: This article first appeared in the October 2023 issue of Light Metal Age. To receive the current issue, please subscribe.