Editor’s Note: The focus of this issue’s patent survey is a comparison of primary aluminum invention patents granted in both the U.S. and China in just the past year. In both countries, the issued invention patents are enforceable over a 20-year term. At the same time, the contrast in the present state of primary aluminum production between the U.S. and China is striking, with China operating over 100 newer energy-efficient smelters that produced some 37 million tonnes in 2020 (almost 60% of global output) compared with with six older smelters in the U.S. that produced one million tonnes in 2020. The newest U.S primary smelter opened in 1980, while China accounted for 119 of the 133 aluminum smelters built globally between 1985 and 2005, according to the London Metal Exchange. Still, Chinese demand for aluminum outpaces its productive capacity, making China in 2020 and in early 2021 a net importer of primary aluminum and its alloys.
It is interesting to note that, of the U.S. patents granted last year and selected here on primary aluminum technology, the majority were assigned to foreign companies. The selected Chinese patents in the last year were all assigned to Chinese companies, mostly Aluminum Corporation of China, Ltd. Although, three of these U.S. patents were granted to the Elysis Limited joint venture in Canada, which comprises Alcoa, Rio Tinto, and the governments of Canada and Quebec. These recent U.S. patents involve radical changes to the conventional aluminum electrolysis production method, including the use of vertically positioned inert anodes and wettable cathodes. The Chinese patents of last year aim to improve productivity of aluminum smelters in place throughout China (in 2014, the aluminum smelting power consumption reference published by China Ministry of Industry and Information Technology was 12,500 KWh/tonne) and, also, some of these patents deal with environmental issues. It should be noted that innovative primary aluminum technologies, such as inert anodes and wettable cathodes, have been the subject of several previously published Chinese invention patents. Also, readers might look over the LMA patent survey published in February 2020 (http://lma.bz/feb2020pats), which focused on inert anodes and wettable cathodes.
Projections by the IAI for future global demand for primary aluminum show a 40% or more increase through to 2050 due to a growing need for environmental and sustainable solutions in transportation, B&C, infrastructure, energy, and food security that aluminum fulfills. As an example, the new U.S. fuel economy proposal will raise the 2026 Corporate Average Fuel Economy standard to 48 mpg from the current 40 mpg. Some of the timely patents presented here will probably find application in achieving these goals.
— Joseph C. Benedyk, Editor
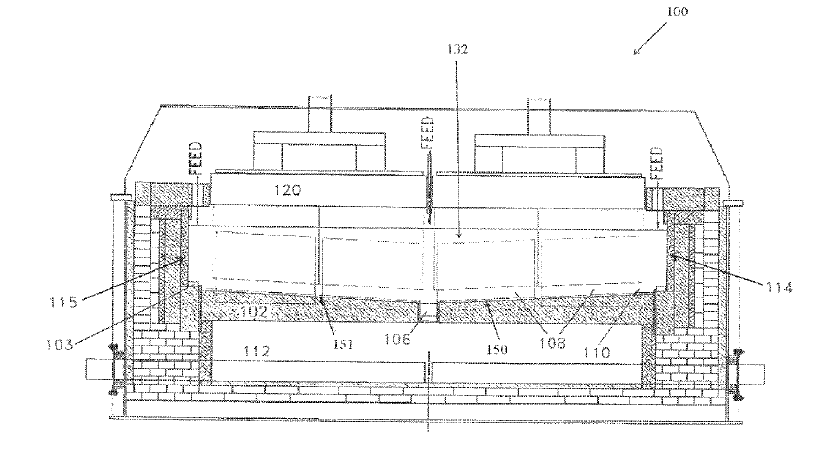
US11203814 — APPARATUSES AND SYSTEMS FOR VERTICAL ELECTROLYSIS CELLS — Alcoa USA Corp. (USA) — In one embodiment, the disclosed subject matter relates to an electrolytic cell that has: a cell reservoir; a cathode support retained on a bottom of the cell reservoir, wherein the cathode support contacts at least one of: a metal pad and a molten electrolyte bath within the cell reservoir, wherein the cathode support includes: a body having a support bottom, which is configured to be in communication with the bottom of the electrolysis cell; and a support top, opposite the support bottom, having a cathode attachment area configured to retain a at least one cathode plate therein, wherein the cathode support comprises at least one cathode attachment configured to hold the plurality of vertical cathode plates in a vertical orientation; a plurality of vertical anode plates (carbon based or inert anodes) adjacent to and overlapping with the plurality of vertical cathode plates, wherein the plurality of vertically anode plates are at least partially disposed within the shell.
US11186897 — METHOD FOR PRODUCING ALUMINUM-SCANDIUM ALLOY AND REACTOR FOR IMPLEMENTING THE METHOD — United Company RUSAL Engineering and Technology Centre, LLC (Russia) — Disclosed herein are methods for producing an aluminum-scandium alloy comprising 0.41-4 wt % of scandium which can be used in industrial production setting. The method is carried out by melting aluminum and a mixture of salts comprising sodium, potassium and aluminum fluorides followed by performing simultaneously, while continuously supplying scandium oxide, an aluminothermic reduction of scandium from its oxide and an electrolytic decomposition of the formed alumina. Periodically, at least a portion of the produced alloy is removed, aluminum is then charged, and the process of alloy production is continued while supplying scandium oxide. Also disclosed is a reactor for producing an aluminum-scandium alloy pursuant to the methods described herein.
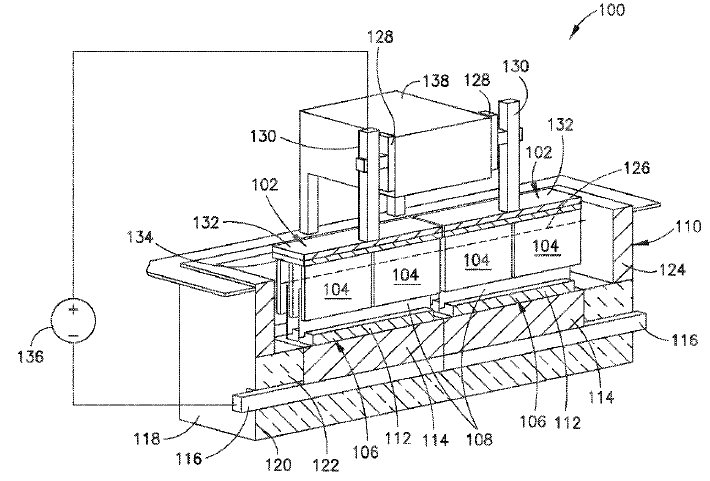
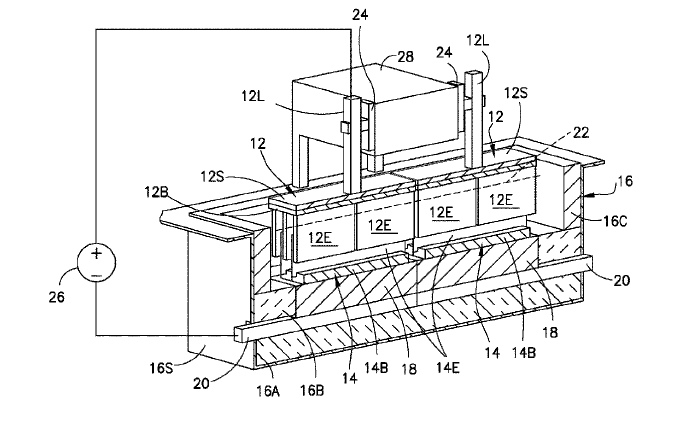
US11180862 — ADVANCED ALUMINUM ELECTROLYSIS CELL — Elysis Limited Partnership (Canada) — In some embodiments, an electrolytic cell includes: an one anode module having a plurality of anodes; a one cathode module, opposing the anode module, and comprising a plurality of vertical cathodes, wherein each of the plurality of anodes and each of the plurality of vertical cathodes are vertically oriented and spaced one from another; a cell reservoir; and a cell bottom supporting the cathode module, wherein the cell bottom comprise an first upper surface, a second upper surface, and a channel, wherein the plurality of vertical cathodes extends upward from the upper surfaces, wherein at least one cathode block is located below the plurality of vertical cathodes, wherein the first upper surface and the second upper surface are configured to direct substantially all of the liquid aluminum produced in the electrolytic cell to the channel, and wherein the channel is configured to receive liquid aluminum from the upper surfaces. The aluminum wettable material of the cell bottom is at least one of TiB2, ZrB2, HfB2, SrB2, or combinations thereof.
US11120948 — ELECTROLYTE FOR ALUMINUM ELECTROLYTIC CAPACITOR AND ALUMINUM ELECTROLYTIC CAPACITOR USING ELECTROLYTE — Shenzhen Capchem Technology Co., Ltd. (China) — An electrolyte for an aluminum electrolysis capacitor and the aluminum electrolysis capacitor using the electrolyte are provided. The electrolyte comprises a primary solute, a primary solvent, and an additive as shown in a structural formula 1, wherein, R1 and R2 are each independently selected from –CH3, –CH3CH3 or –OH; R3 and R4 are each independently selected from –(CH2CH¬2O)mH or –H, and n and m are integrals ranging from 1 to 10,000, respectively, as shown in Figure 1. Further, content of the above additive in the above electrolyte is 5%-10% of the total mass of the above electrolyte. The electrolyte has excellent anti-corrosive performance and is capable of maintaining a long load service life under the condition of relatively high chlorine ion content, and there is no evidence of corrosion in the capacitor after it is disassembled.
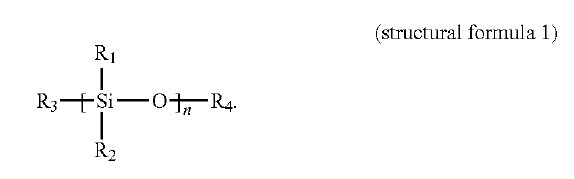
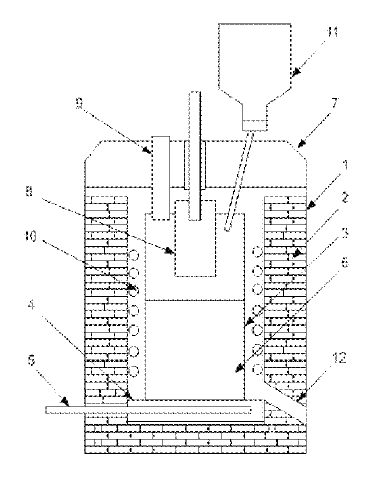
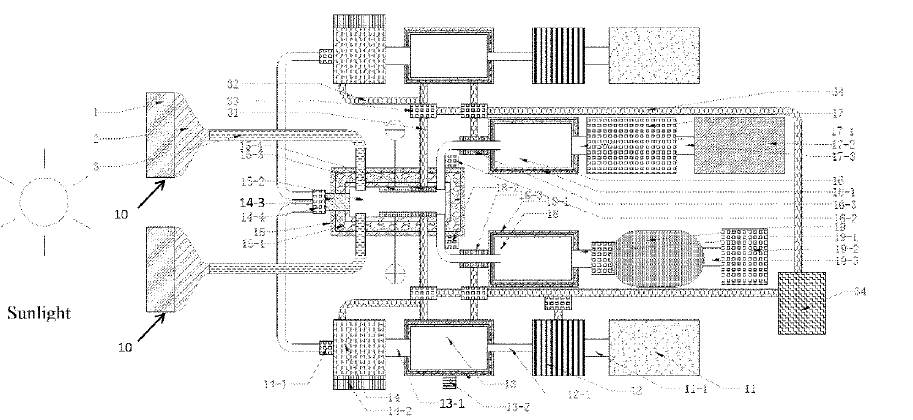
US11098388 — ALUMINUM HYDROXIDE SOLAR POWERED THERMAL REDUCTION DEVICE FOR ALUMINUM-AIR FUEL CELL — Lanzhou Jinfule Biotechnology Co. Ltd. (China) and Spring Power Limited (British Virgin Islands) — The present invention provides an apparatus for preparing a metal based on solar energy thermal reduction. The apparatus includes a solar energy collection and photothermal conversion system and a thermal reduction system. The solar energy collection and photothermal conversion system includes: a solar energy collection device (1), a solar energy concentration device (2), and a solar energy transfer device (3) or a photothermal conversion and transfer device. The thermal reduction system includes: a metal reduction chamber (15), an electric field and/or magnetic field generation device (15-3), and a separation and cooling device (15-4). The solar energy collection and photothermal conversion system transfers sunlight or heat to the metal reduction chamber to decompose a metal oxide, and a product resulted from the decomposition is dissociated under the effect of an electric field/magnetic field, and a liquid metal is obtained upon cooling. The apparatus further includes a waste heat recovery and recycle system. According to the present invention, the metal oxide is heated and decomposed by directly using the solar energy, which improves energy utilization rate, greatly prevents environmental pollution and energy waste, and has a great application prospect.
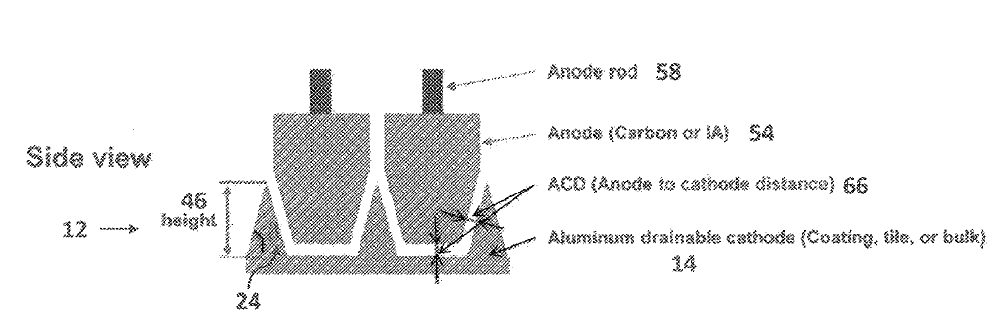
US11078584 — SYSTEMS AND METHODS OF ELECTROLYTIC PRODUCTION OF ALUMINUM — Alcoa USA Corp. (USA) — In some embodiments, an exemplary electrolytic cell includes: a wettable cathode structure disposed within an electrolysis cell, wherein the electrolysis cell is configured to produce metal on a surface of the cathode structure, wherein the cathode structure is configured to fit along a floor of the electrolysis cell, wherein the cathode structure has a sloped surface when compared to a generally horizontal plane, and wherein via the sloped surface, the cathode structure is configured to drain a metal product from the sloped surface towards a lower end of the cathode structure.
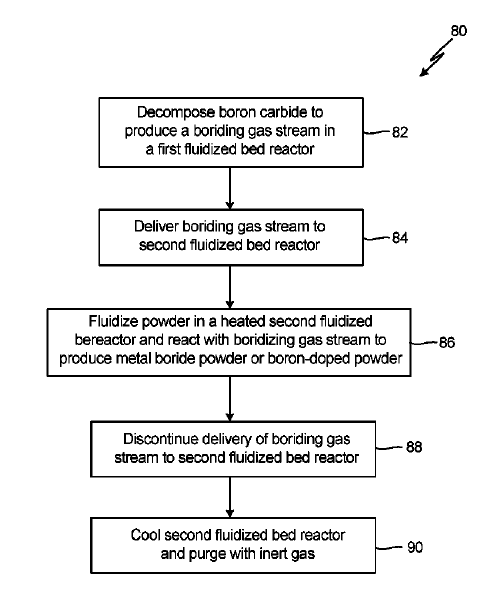
US11066308 — PREPARATION OF METAL DIBORIDE AND BORON-DOPED POWDERS — United Technologies Corporation (USA) — Titanium diboride (among other metal and alloy borides) is a promising material for engineering applications including abrasive and cutting tools, wear-resistance coating, cemented carbide, cathodes for aluminum electrolysis cells, and crucibles for holding molten metals. A method for producing a metal boride powder includes producing a bonding gas stream from a first powder in a first fluidizing bed reactor, delivering the bonding gas stream to a second fluidized bed reactor through a conduit fluidly connecting the first and second fluidized bed reactors, fluidizing a second powder in the second fluidized bed reactor, mixing the second powder with the bonding gas stream such that a metal boride or boron-doped powder is formed.
US11060199 — ELECTRODE CONFIGURATIONS FOR ELECTROLYTIC CELLS AND RELATED METHODS — Elysis Limited Partnership (Canada) — In one embodiment, an electrolytic cell for the production of aluminum from alumina includes: at least one anode module having a plurality of inert anodes; at least one cathode module, opposing the anode module, wherein the at least one cathode module comprises a plurality of cathodes, wherein the plurality of anodes are suspended above the cathode module and extending downwards towards the cathode module, wherein the plurality of cathodes are positioned extending upwards towards the anode module, wherein each of the plurality of anodes and each of the plurality of cathodes are alternatingly positioned, wherein the plurality of anodes is selectively positionable in a horizontal direction relative to adjacent cathodes, wherein the anode module is selectively positionable in a vertical direction relative to the cathode module, and wherein a portion of each of the anode electrodes overlap a portion of adjacent cathodes.
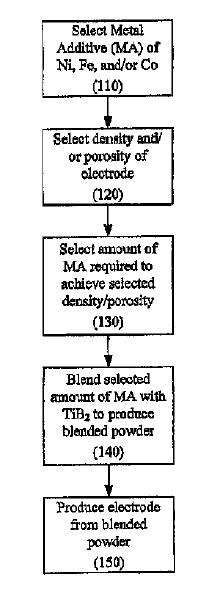
US11041250 — COMPOSITION FOR MAKING WETTABLE CATHODE IN ALUMINUM SMELTING — Alcoa USA Corp. (USA) — Compositions for making wettable cathodes to be used in aluminum electrolysis cells are disclosed. The compositions generally include titanium diboride (TiB2) and metal additives comprising up to 10 wt. % metal additives which at least include Cr and may further include Mn, Mo, Pt, Pd Fe, Ni, Co, and W. The amount of selected metal additives may result in production of electrodes having a tailored density and/or porosity. The electrodes may be fabricated from selected powder compositions using powder sintering processes such as hot pressing or sintering at atmospheric pressure at temperatures of about 1400° C to about 2100° C. The electrodes may be durable and used in aluminum electrolysis cells.
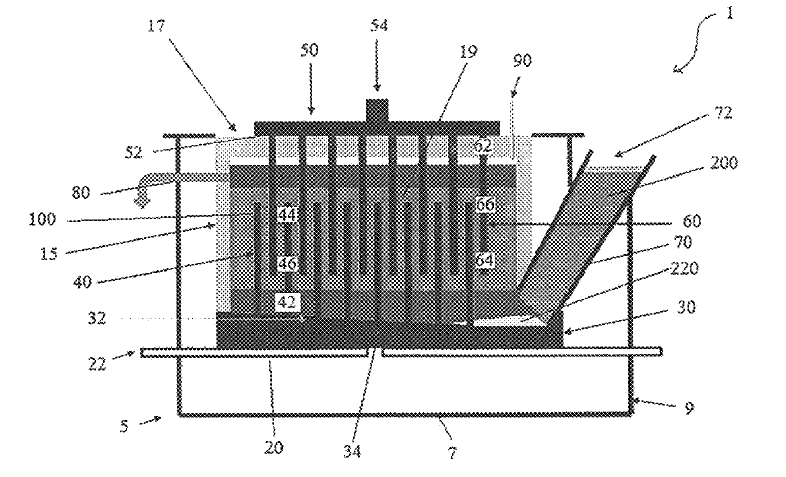
US11001931 — SYSTEMS AND METHODS FOR PURIFYING ALUMINUM — Alcoa USA Corp. (USA) — The application is directed towards methods for purifying an aluminum feedstock material. More specifically, the application is directed towards utilizing vertically oriented, interspaced anode and cathode configuration, where the anodes and cathodes are configured from aluminum-wettable material, in order to reduce inter-polar distance, and increase the electrode surface area (e.g. purification zone) of an electrolysis cell operating to produce purified aluminum metal product from an aluminum feedstock with much lower energy consumption and higher productivity/ A method provides: (a) feeding an aluminum feedstock into a cell (b) directing an electric current into an anode through an electrolyte and into a cathode, wherein the anode comprises an elongate vertical anode, and wherein the cathode comprises an elongate vertical cathode, wherein the anode and cathode are configured to extend into the electrolyte zone, such that within the electrolyte zone the anode and cathode are configured with an anode-cathode overlap and an anode-cathode distance; and producing some purified aluminum product from the aluminum feedstock.
US10982342 — DEVICE AND METHOD FOR DETERMINING THE COMPOSITION OF AN ELECTROLYTE — United Company RUSAL Engineering and Technology Centre LLC (Russia) — This invention relates to nonferrous metallurgy, particularly to a device and method for electrolyte composition analysis based on differential thermal measurements for aluminum electrolysis control. The device is comprised of a metal body including a reference material and an electrolyte sample receptacle, temperature sensors immersed into the reference material and in an electrolyte sample, a system for registration, data processing, and visualization of obtained results. A method includes immersing a metal body into an electrolyte; filling a receptacles with the molten electrolyte; removing and cooling down the metal body having the filled receptacle above a crust on the molten electrolyte surface; drawing and analyzing differential-thermal curves based on which the liquidus temperature, electrolyte superheating and phase and blend compositions of electrolyte solid samples are determined taking into account all crystallizing phases the content of which in the electrolyte sample is no less than 3 wt %.
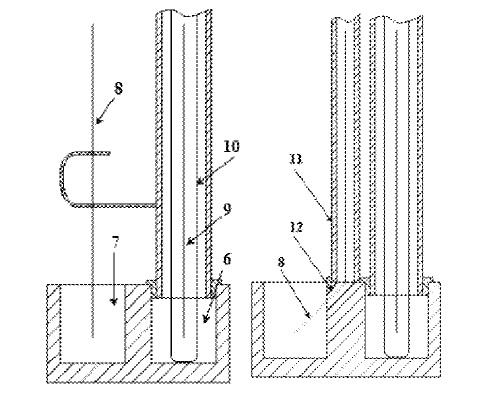
US10975484 — ELECTROLYTE FOR OBTAINING MELTS USING AN ALUMINUM ELECTROLYZER — United Company RUSAL Engineering and Technology Centre LLC (Russia) — The invention concerns non-ferrous metallurgy, in particular the composition of an electrolyte for electrically obtaining aluminum by the electrolysis of fluoride melts. The electrolyte proposed contains, in % by weight: sodium fluoride 26-43, potassium fluoride up to 12, lithium fluoride up to 5, calcium fluoride 2-6, alumina 2-6, aluminum fluoride and admixtures the remainder. The technical result is to increase the solubility of alumina in the electrolyte at a temperature of 830-930° C. In the electrolyte being applied for, the carbon and inert electrode materials are not destroyed, and the use of special methods to purify the aluminum of melt components is not required.
US10920329 — ANODE ASSEMBLY FOR ALUMINUM ELECTROLYSIS CELLS AND METHOD FOR MANUFACTURING ANODE ASSEMBLIES — Universite du Quebec Chicoutimi (Canada) — An anode assembly for an aluminum electrolysis cell is provided. The anode assembly includes a baked anode block, a plurality of elongated connection elements each having an anode block contact surface and an electrical connection surface, at least one electromechanical crossbar connector covering the electrical connection surfaces of the elongated connection elements, and a crossbar electrically connected to the elongated connection elements. A method for manufacturing an anode assembly for an aluminum electrolysis cell is also provided. The method includes the steps of forming a block of green anode paste, inserting a plurality of elongated connection elements in the green anode paste, baking the green anode, positioning a crossbar above the electrical connection surfaces of the plurality of elongated connection elements, and covering the electrical connection surfaces and at least partially the crossbar with a surface-conforming electrically conductive material.
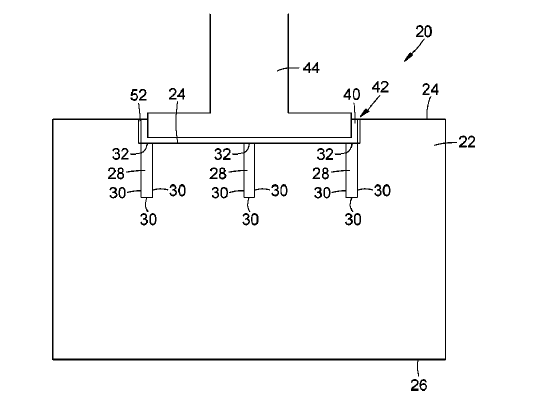
US10801118 — ELECTROLYSIS CELL, IN PARTICULAR FOR THE PRODUCTION OF ALUMINUM — Tokai Cobex GMB (Germany) — An electrolysis cell, for production of aluminum, contains a cathode, a layer of liquid aluminum arranged on the upper side of the cathode, a melt layer thereon and an anode on the top of the melt layer. The cathode is composed of at least two cathode blocks, wherein at least one of the at least two cathode blocks differ from at least one of the other cathode blocks in the average compressive strength, the average thermal conductivity, the average specific electrical resistivity and/or the apparent density. This allows to at partially homogenize the wear profile, which is formed during the electrolysis, across the surface of the cathode by homogenizing the rate of mechanical abrasion, the electrical current density and/or the temperature profile across the surface of the cathode by simply arranging different cathode blocks with appropriate properties together. Thus, the energy efficiency, the lifetime, the stability as well as the reliability of specifically the cathode and in general of the electrolysis cell are improved.
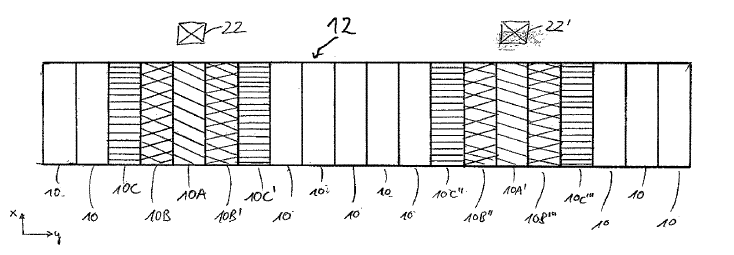
US10711359 — IRON-BASED ANODE FOR OBTAINING ALUMINUM BY THE ELECTROLYSIS OF MELTS — United Company RUSAL Engineering and Technology Centre LLC (Russia) — The invention concerns non-ferrous metallurgy, particularly an anode for electrolytically obtaining aluminum by the electrolysis of fluoride melts. The anode for obtaining aluminum by means of the electrolysis of melts at a temperature of less than 930°C. consists of a base executed of an alloy containing 65-96%wt of iron, less than 35%wt of copper, less than 20%wt of nickel, and one or several additives from molybdenum, manganese, titanium, tantalum, tungsten, vanadium, zirconium, niobium, chromium, aluminum (less than 1%wt) cobalt, cerium, yttrium, silicon, and carbon totaling less than 5%, and a protective oxide layer comprising iron oxides and complex oxides of iron, copper, and nickel. The protective oxide layer on the anode surface is obtained by preliminary oxidation in air at a temperature of 850-1050°C. or subsequently in the electrolysis process by oxidation with oxygen evolving at the anode.
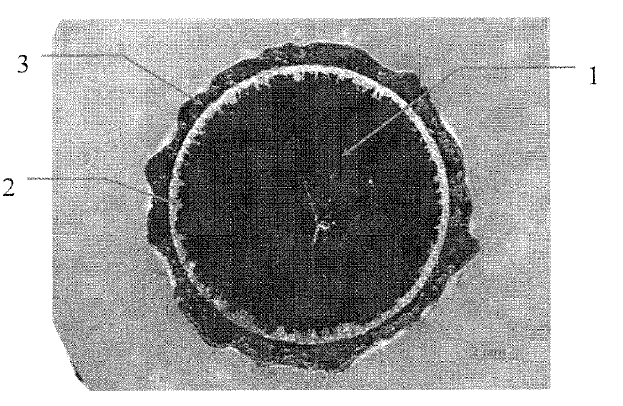
US10697074 — ELECTROLYTIC CELL INTENDED FOR THE PRODUCTION OF ALUMINIUM AND ELECTROLYTIC SMELTER COMPRISING THIS CELL — Rio Tinto Alcan International Limited (Canada) — This cell (1) comprises a pot shell (2) having two longitudinal sides (18) which are symmetrical in relation to a longitudinal median plane (P) of the electrolytic cell (1), an anode assembly which can only move in vertical translation with respect to the pot shell (2), the anode assembly comprising an anode block (100) and a transverse anode support (200) extending at right angles to the longitudinal sides (18) of the pot shell (2), from which support the anode block (100) is suspended. The anode support (200) comprises two connecting portions (202) from which electrolysis current is supplied to the anode support (200), and the cell (1) comprises electrical connection conductors (20) electrically connected to the two connecting portions (202) of the anode support (200), the two connecting portions (202) being located on either side of the plane (P).
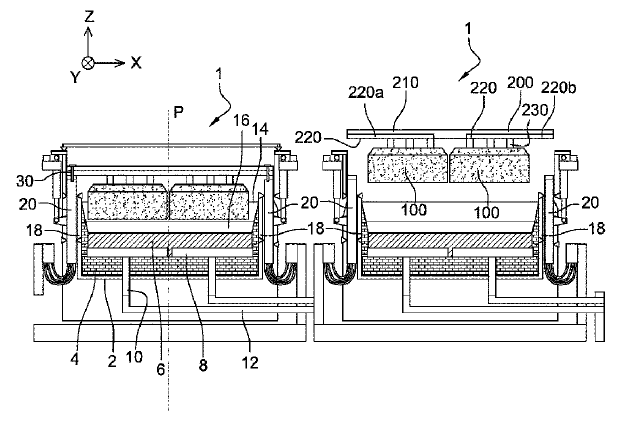
US10513788 — ELECTROLYSIS TANK COMPRISING AN ANODE ASSEMBLY CONTAINED IN A CONTAINMENT ENCLOSURE — Rio Tinto Alcan International Limited (Canada) — A cell includes a pot shell defining an opening through which an anode block is designed to be moved, said anode block being suspended from an anode support forming with said anode block an anode assembly mobile in relation to the pot shell, and a confinement chamber defining a closed volume above said opening for the containment of the gases generated during the production of aluminum, the anode support being connected to an electrical conductor to supply an electrolysis current to the anode block, the anode assembly is fully contained in the confinement chamber, and in that the electrical connection between the mobile electrical conductor and the anode support is made within the confinement chamber.
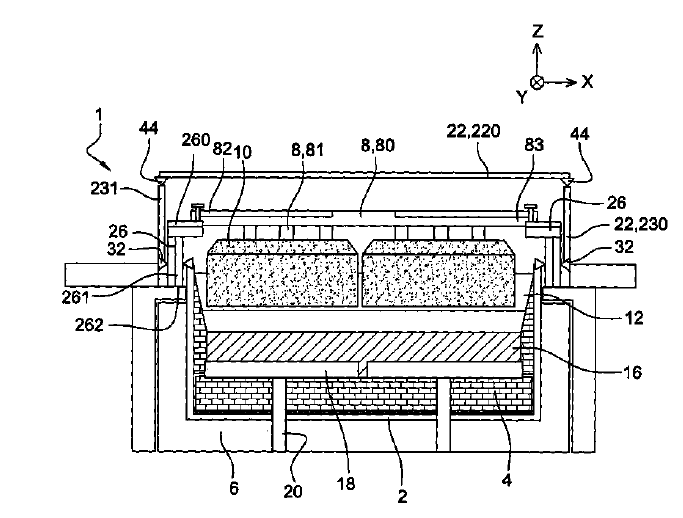
US10415122 — CERMET ELECTRODE MATERIAL — Elysis Limited Partnership (Canada) — The invention relates to electrode materials, particularly cermet materials used in the composition of anode material for production of aluminum by electrolysis. It relates more specifically to cermet materials used for the manufacture of so-called “inert” or “non-consumable” anodes. Cermet materials include as mass percentages, at least: 50% to 90% of a metallic phase containing an alloy of copper (Cu) and nickel (Ni), and 10% to 50% of an oxide phase containing at least iron, nickel, and oxygen with the following proportion by mass of Ni: 0.2%≤Ni≤17%. An electrode, preferably an anode, may include this cermet material.

CN113324418 (A) — ALUMINUM ELECTROLYSIS WASTE CATHODE — Aluminum Corporation of China Ltd. (China) — The embodiment of the invention provides an aluminum electrolysis waste cathode treatment device and method. The device comprises a furnace body, a first electrode assembly, a second electrode assembly and a power supply assembly, wherein the top of the furnace body is provided with a feeding port, and the bottom of the furnace body is provided with a discharging port; at least part of the first electrode assembly is arranged in the furnace body, and the first electrode assembly is located above the furnace body; part of the second electrode assembly is arranged in the furnace body, and a material treatment space is formed between the second electrode assembly and the first electrode assembly; and the power supply assembly is connected to the first electrode assembly and the second electrode assembly. According to the aluminum electrolysis waste cathode treatment device, heating is conducted through resistors of waste cathode materials and electric arcs generated by mutual collision between the waste cathode materials, the stability of a temperature can be guaranteed, carbon substances obtain uniform energy, graphite products are uniform, and the furnace body can be always kept at a high temperature of 2500° C or above, so that the utilization efficiency of aluminum electrolysis waste cathodes and the quality of the graphite products can be improved.
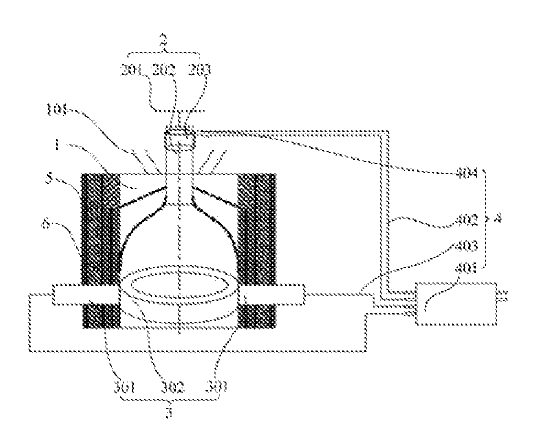
CN113237907 (A) — METHOD FOR MEASURING FLUORINE CONTENT IN FLUORINE-LOADED ALUMINUM OXIDE BY X-RAY FLUORESCENCE SPECTROMETRY — Aluminum Corporation of China Ltd. (China) — The invention particularly relates to a method for measuring the fluorine content in fluorine-loaded aluminum oxide by X-ray fluorescence spectrometry, which belongs to the technical field of metal content detection. The method provided by the invention is suitable for measuring the fluorine content in fluorine-loaded aluminum oxide with the mass percentage content of F being 0.1%-5%. According to the method, the fluorine content in the fluorine-loaded aluminum oxide can be rapidly and accurately measured, high-temperature melting of the sample is not needed in the measurement process, and the problem of fluorine volatilization in the high-temperature melting process is effectively solved. The method is easy and convenient to operate and high in accuracy, the detection process and period are greatly shortened, the detection efficiency is improved, labor cost and economic cost are saved for enterprises in detection, and high economic benefits are achieved; meanwhile, a detection method is provided for aluminum electrolysis enterprises to evaluate the fluorine-loaded capacity and the flue gas purification effect of fluorine-loaded aluminum oxide, and the method has wide application prospects in the aspects of environmental protection and evaluation.
CN112845518 (A) — HIGH-VALUE UTILIZATION METHOD OF CARBON RESIDUES — China Aluminum Environmental Protection and Energy Conservation Group Co. Ltd. (China) — The invention discloses a comprehensive utilization method of carbon residues. The method comprises the following steps: primarily separating carbon materials from non-carbon materials in the carbon residues by adopting a wet process floatation process to obtain carbon powder and electrolyte; adding additives into the electrolyte to obtain a mixture, uniformly mixing the mixture, then roasting the mixture in a medium-low-temperature roasting kiln to prepare composite materials; pulverizing the composite materials and then washing the composite materials with water to obtain washing liquid and a filter cake; drying the filter cake and returning to an electrolytic bath; concentrating the washing liquid and performing electrolysis by using a bipolar membrane electrodialysis method to prepare acid and alkali; enabling the prepared alkali to enter a Bayer system to produce aluminum oxide, and ensuring that the prepared acid is used for leaching out carbon powder so that the purity of the carbon powder is improved. The method disclosed by the invention has the characteristics that a production process is convenient to control, industrialized stable production is easy, a product added value is high and zero emission of wastes is realized.
CN112765894 (A) — ALUMINUM ELECTROLYSIS CELL STATE PREDICTION METHOD BASED ON K-LSTM — North China University of Technology (China) — The invention discloses an aluminum electrolysis cell state prediction method based on K-LSTM. The method comprises the following steps that 1, conducting normalization processing on data; 2, constructing a training set and a test set according to a set sliding window size m; 3, constructing an improved LSTM model and initializing the parameters of the model; 4, training a prediction model by using the training set, updating parameters by adopting a gradient descent method, and iterating for several times until the precision requirement is met; and 5, feeding the test set to the trained model, and obtaining a predicted value at the t+1 moment by using historical data. Based on the improved K-LSTM algorithm, aiming at the problem of sample imbalance existing in an LSTML forgetting gate unit, the sample imbalance is eliminated through a weight setting method, and the state of the aluminum electrolysis cell can be effectively predicted.
CN112048740 (A) — SYSTEM AND METHOD FOR IMPROVING CONTROL SAFETY OF ALUMINUM ELECTROLYSIS CELL CONTROLLER — Aluminum Corporation of China Ltd. (China) — The invention discloses a system and method for improving the control safety of an aluminum electrolysis cell controller. The method comprises the following steps that an anode lifting command on thecell controller is detected and compared, the action direction of an anode bus is monitored in real time, an anode ascending instruction is compared with a positive and negative rotation signal and a moving signal, it is indicated that the anode action is normal if consistency is achieved, and if not, it is indicated that the anode action is abnormal; and the anode descending instruction is compared with the positive and negative rotation signal and the moving signal, it is indicated that the anode action is normal if consistency is achieved, if not, it is indicated that the anode action is abnormal. Through monitoring, slot pulling and slot pressing safety accidents caused by lifting contactor adhesion, phase sequence errors, wiring errors or manual operation errors are avoided, the control safety of the cell controller is improved, the method can accurately judge the control abnormity of the cell controller, discover possible hidden dangers of an electrolytic cell as soon as possible, prevent accidents in real time, and fundamentally improve the control safety of the cell controller from closed-loop detection of the first link and the last link of anode lifting control.
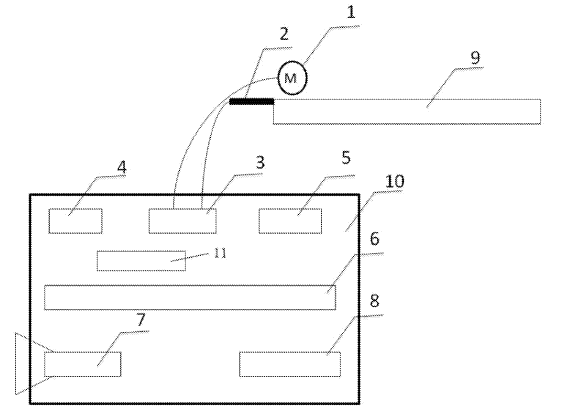
CN112048739 (A) — SAFETY PRE-WARNING METHOD AND SAFETY PRE-WARNING SYSTEM FOR ALUMINUM ELECTROLYSIS CELL — Aluminum Corporation of China Ltd. (China) — The invention discloses a safety pre-warning method for an aluminum electrolysis cell. The safety pre-warning method includes the following steps of: determining more than one type of temperature measurement area on the aluminum electrolysis cell, determining more than two area position identifiers on each type of temperature measurement area, and determining corresponding identifier areas according to the area position identifiers; carrying out infrared thermal imaging photographing on each type of temperature measurement area to obtain temperature distribution data of each temperature measurement area; judging whether an over-temperature point exceeding the high-limit threshold value of temperature of the corresponding area exists in each type of temperature measurement area or not; if yes, outputting safety warning information; judging whether the temperature of each identifier area at the current moment is in a rising trend and exceeds a rising threshold value or not in comparison with the temperature before the current moment and within a preset time period; if yes, outputting safety pre-warning information; judging whether the temperature deviation between each identifier area and the adjacent identifier area exceeds a deviation threshold value or not; and if yes, outputting safety reminding information. The safety pre-warning method can effectively prevent furnace leakage of the electrolysis cell and is beneficial to safe and stable production of the electrolysis cell.
CN112033566(A) — DISTRIBUTED OPTICAL FIBER TEMPERATURE MEASUREMENT SYSTEM FOR ALUMINUM ELECTROLYSIS CELL — China Aluminum Intelligent Tech Development Co. Ltd . (China) — The invention discloses a distributed optical fiber temperature measurement system for an aluminum electrolysis cell. The system comprises a temperature measurement device, a temperature measurement optical fiber and a temperature monitoring system, and is characterized in that the temperature monitoring system is connected with the temperature measurement device, the temperature measurement optical fiber comprises a high-temperature temperature sensing optical fiber and a low-temperature temperature sensing optical fiber, the low-temperature temperature sensing optical fiber is arranged at the bottom of the electrolysis cell, and the high-temperature temperature-sensing optical fiber is arranged at the side part of the electrolytic cell. The substantive effects of the system are that the temperature measuring device emits a laser signal and returns the laser signal through the temperature measuring optical fiber to form a closed loop, each temperature measuring point is accurately positioned by detecting the return time of the optical signal passing through different temperature measuring points, and the temperature of each temperature measuring point is detected by analyzing change of Rayleigh lines and Raman lines so as to detect the temperature of the bottom or side part of the aluminum electrolysis cell, and the temperature of each point is visually and continuously displayed in the temperature monitoring system, and the abnormal temperature is alarmed. Effective data guarantee is provided for aluminum electrolysis production operation and operation guidance, the production stability can be effectively improved, and the occurrence rate of cell leakage can be effectively reduced.
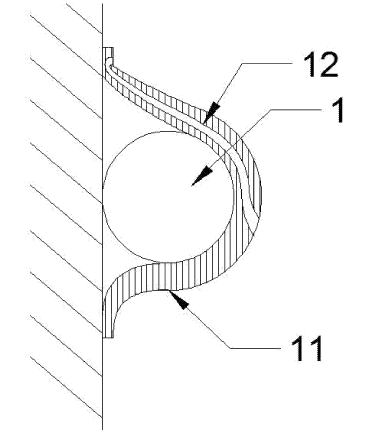
CN112014265 (A) — CONTINUOUS ANODE ALUMINUM ELECTROLYSIS CELL ANODE PASTE PERFORMANCE EVALUATION DEVICE AND EVALUATION METHOD — Aluminum Corporation of China Ltd. (China) — The invention relates to a device and a method for evaluating the performance of anode paste of a continuous anode aluminum electrolysis cell. A mold of the device is provided with a cavity for containing the anode paste, the top of the cavity is open, a pressing block can freely slide into the cavity from the top of the cavity in the vertical direction, an exhaust hole is formed in the pressing block, the exhaust hole is communicated with the cavity, and a heating assembly is connected to the mold and used for heating the mold. According to the invention, by acquiring corresponding height values of the anode paste at normal temperature, stable viscosity temperature of the anode paste and roasting temperature and by obtaining the height value at the corresponding temperature, the performance of the anode paste of the continuous anode aluminum electrolysis cell is obtained, the corresponding height values of the anode paste at the normal temperature, the stable viscosity temperature of the anode paste and the roasting temperature are convenient and rapid to obtain, the operation is simple, the detection efficiency is very good, and the detection requirement of the performance of the continuous anode material can be met.
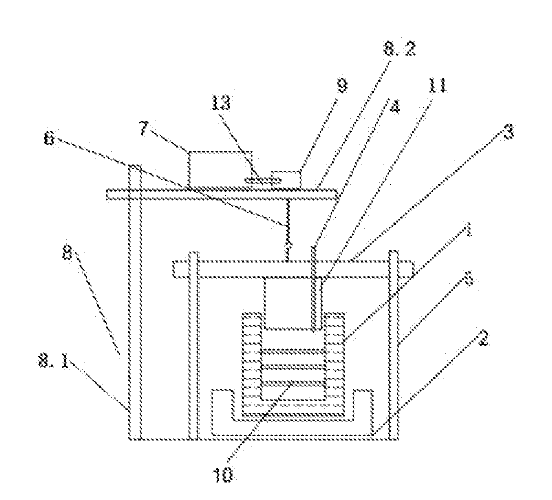
CN111996556 — ALUMINUM ELECTROLYSIS CELL ENERGY BALANCE CONTROL SYSTEM — Aluminum Corporation of China Ltd. (China) — The invention discloses an aluminum electrolysis cell energy balance control system, which comprises a temperature acquisition assembly, a first control assembly and a second control assembly, wherein the temperature acquisition assembly is used for acquiring temperature data of M fire holes in an aluminum electrolysis cell after crust breaking; the first control assembly judges whether there exist fires holes in the M fire holes that are blocked or not in the selected time period according to the real-time temperature change rate of the temperature data, calculates an average temperature change rate Kij of each unblocked fire hole in a preset time interval after each crust breaking, and a temperature change trend Rj of each unblocked fire hole in a selected time period determined according to the Kij, and determines and outputs thermotaxis stroke information or cold-taxis stroke information of the aluminum electrolysis cell according to the Rj; and the second control assembly controls the energy balance of the aluminum electrolysis cell according to the aluminum electrolysis cell thermotaxis stroke information or the aluminum electrolysis cell cold taxis stroke information. The control system can feed back and adjust the energy balance state of the electrolytic cell timely and accurately.
CN111945187 — METHOD FOR REDUCING ANODE GROSS DISSIPATION OF PREBAKED ALUMINUM ELECTROLYSIS CELL — Aluminum Corporation of China Ltd. (China) — The invention discloses a method for reducing the anode gross dissipation of a prebaked aluminum electrolysis cell. The method comprises the following steps: preparing a plurality of anodes, and measuring the first height H1 of each anode; replacing the anode scrap of the prebaked aluminum electrolysis cell with the plurality of anodes, after using the anodes for n days, measuring the consumption height delta H of each anode, and obtaining the second height H2 of each anode according to the first height H1 and the consumption height delta H; obtaining the minimum value H2min of the second height; obtaining the daily average consumption height of each anode (refer to the Specification), and adjusting the service time of each anode according to the second height H2, the second height minimum value H2min and the daily average consumption height (refer to the Specification) of each anode; if (refer to the Specification), not prolonging the service time of each anode; if(refer to the Specification), prolonging the service life of each anode, and the prolonged service life is N * 8h, and (refer to the Specification).
CN111892022 (A) — ALUMINUM ELECTROLYSIS FLUORINE-CONTAINING WASTE AND SURPLUS ELECTROLYTE RESOURCE UTILIZATION METHOD — Aluminum Corporation of China Ltd. (China) — The invention discloses an aluminum electrolysis fluorine-containing waste and surplus electrolyte resource utilization method. The method comprises the following steps: crushing and levigating the aluminum electrolysis fluorine-containing waste and surplus electrolyte to prepare electrolyte powder, adding the electrolyte powder and an acid into a negative pressure reactor for reaction, condensing and rectifying hydrogen fluoride generated by the reaction to obtain a hydrofluoric acid product, adding a neutralizer into reaction residual liquid for treatment, and filtering to obtain filter residues which are calcium salt and a filtrate which is a sodium aluminate solution for aluminum oxide production. According to the method, full-quantitative resource utilization of the aluminum electrolysis fluorine-containing waste and the surplus electrolyte can be achieved, the utilization process flow is short, no secondary pollutants are generated, the obtained product can be applied to multiple industries, a large resource cycle is formed, meanwhile, new raw materials are provided for the fluorine chemical industry, and positive contribution is made to promotion of development of the fluorine chemical industry.
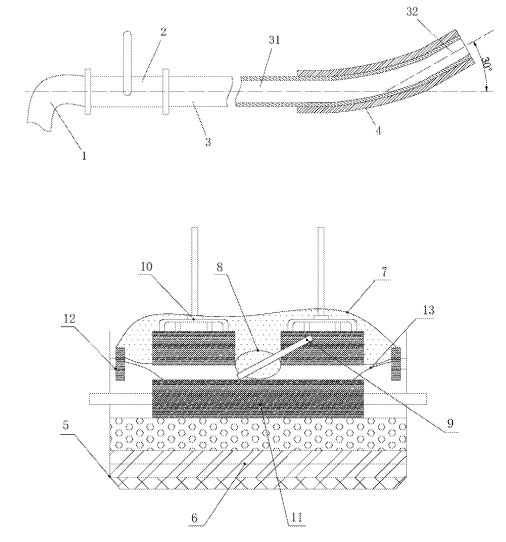
CN111778523 (A) — DEVICE AND METHOD FOR CONTROLLING QUENCHING OF ANODE EFFECT OF ALUMINUM ELECTROLYSIS — Aluminum Corporation of China Ltd. (China) — The invention discloses a device and method for controlling quenching of an anode effect of aluminum electrolysis. The device comprises a metal pipe and a guide-in wind pipe, wherein the metal pipe is connected with the guide-in wind pipe, and the guide-in wind pipe is connected with a wind source; the front section of the metal pipe is a section of curved pipe, through the section of curved pipe, the entire metal pipe is formed into an upwards-tilted structure, and the section of curved pipe is a portion used for extending into an electrolytic cell; and at least the surface of the curved pipe is provided with an insulating layer. The method comprises the following steps of inserting the curved pipe of the metal pipe to the position below an anode at one side, guiding in compressed-air wind, blowing an aluminum liquid and an electrolyte solution in the electrolytic cell through the compressed-air wind to boil the aluminum liquid and the electrolyte solution to promote the escape of bubbles on the anode, so as to realize quenching of the anode effect. The device and the method have the advantages that the device is simple, the operation is convenient, and the cost is low; the current efficiency loss of the anode effect quenching process is greatly reduced; a wood stick does not need to be used, so that the production cost is saved on, and the device and the method are more energy-saving and environment-friendly; and the time for quenching the anode effect is short, and the power consumption of the aluminum electrolysis is saved.
CN111341389 (A) — ELECTROLYTIC ALUMINUM LOAD ELECTRIC HEATING CHARACTERISTIC MODELING METHOD FOR DIRECT LOAD CONTROL — Electric Power Research Institute of State Grid Shandong Electric Power Co. and State Grid Corp. China — The invention discloses an electrolytic aluminum load electric heating characteristic modeling method for direct load control. The electrolytic aluminum load electric heating characteristic modeling method comprises the following steps of building an electrolytic aluminum production series; obtaining an electrolytic aluminum load by adopting a cryolite-aluminum oxide fused salt electrolysis method; establishing an electrolytic aluminum heat dissipating capacity model; establishing an electrolytic aluminum electric heat exchange model; determining electrolytic aluminum load control constraints.
CN110910167 (A) — DATA DRIVING-BASED ECONOMIC SELF-OPTIMIZATION METHOD FOR ALUMINUM ELECTROLYSIS CELL PRODUCTION TECHNOLOGY — Aluminum Corporation of China Ltd. (China) — The invention discloses a data driving-based economic self-optimization method for an aluminum electrolysis cell production technology. The method comprises the following steps: (1) selecting production process technological parameters as electrolysis cell characteristic parameters; (2) according to the electricity price and the aluminum price of an enterprise, calculating the income index of each electrolytic cell according to the aluminum output and the power consumption; (3) carrying out non-guidance classification model operation on the characteristic parameters except the aluminum output and the power consumption of the electrolytic cell to automatically divide the electrolytic cell into 2-3 classes, and calculating an income mean value of each class; (4) selecting an electrolytic cell with the single-cell income index of the electrolytic cell being 10-30% in the first 10-30% from the class with the highest income mean value; (5) averaging the characteristic parameters of the selected electrolytic cells to serve as an optimization target of the process parameters of the same type of electrolytic cells; and (6) according to the sequence of the molecular ratio, the voltage, the aluminum level, the electrolyte level and the aluminum oxide concentration, sequentially adjusting the electrolytic cell parameters with the single-cell income index (I) within the last 30% in the first-class electrolytic cell with the lowest income mean value to the optimized target level. According to the method, the technical economy automatic optimization of multiple parameters of the batch electrolytic cells in different market environments at the same time can be realized.
CN110687148 (A) — METHOD FOR QUANTITATIVELY MEASURING ELEMENT CONTENT OF ALUMINUM ELECTROLYSIS CELL WASTE CATHODE BOTTOM CARBON BLOCK — Aluminum Corporation of China Ltd. (China) — The invention discloses a method for quantitatively measuring element content of an aluminum electrolysis cell waste cathode bottom carbon block. A series of standard samples similar in composition with an overhaul waste cathode bottom carbon block are prepared by adopting various standard samples; sample preparation is carried out by adopting a tablet method; and element content of fluorine, aluminum, sodium, silicon, iron, potassium, calcium, and magnesium in a waste cathode and recovered materials thereof are quantitatively measured by an XRF (X-Ray Fluorescent spectrometry) method. The method is mainly applied to element quantitative measurement in waste cathode harmless resource recovery and utilization. The method solves a problem of influence of an extremely large error of measuring element ingredients of the aluminum electrolysis cell waste cathode on material addition, drug addition, temperature control and the like, and can implement accurate measurement on the element content of the waste cathode and recycled substances thereof so as to reinforce process control in the waste cathode harmless recycling.