By Dr. Anne Deacon Juhl, AluConsult and AnodizingSchool.
Recycled aluminum, when properly processed, can deliver anodized finishes indistinguishable from primary aluminum. While higher levels of trace elements, like zinc (Zn), copper (Cu), and iron (Fe), can increase sensitivity to corrosion or surface defects, these challenges are often smaller than variations caused by production methods and heat treatment. Yet the real obstacle today may not be trace elements themselves, but the availability and quality of recycled material, and how it is handled throughout the production chain.
A paper from Bauger, et al.,1 and recent research from Prof. Rajan Ambat2 have shown that the variation in corrosion resistance within the same alloy — depending on production method, heat treatment, and temper — can be larger than the variation introduced by trace elements alone.This suggests that the industry should focus as much on process control and understanding the alloy’s microstructure as on chemical composition.
This article continues the discussion from the previous article, published in the June 2025 issue of Light Metal Age,3 which explored why recycled aluminum still faces challenges, why these challenges are linked to trace elements, such as Zn, Cu, and Fe, and why the real solution lies not only in cleaning up scrap streams, but also in ensuring that the virgin aluminum used is produced with the lowest possible CO2 footprint. After all, around 75% of all aluminum ever made is still in use — so, the pursuit of 100% recycled material remains a long-term goal. In the meantime, the industry can make a meaningful difference by carefully selecting aluminum produced with renewable or low-carbon energy sources.
Primary Versus Secondary
Although aluminum is one of the most abundant elements on Earth, it is highly reactive, so it does not occur naturally in its pure metallic form. Instead, it is primarily extracted from bauxite ore, which is the main source of alumina (aluminum oxide, Al2O3).
The Bayer process, developed and patented in 1887, remains the dominant industrial method for refining alumina from bauxite, effectively separating it from the iron-rich residue known as red mud. After this refining step, the Hall-Héroult process — discovered independently in 1886 by Charles Martin Hall and Paul Héroult — produces metallic aluminum from alumina through electrolysis.
In this process, alumina is dissolved in a bath of molten cryolite (Na3AlF6), along with various fluoride salts that help control critical parameters, such as bath temperature, density, resistivity, and alumina solubility. Notably, it is this electrolytic reduction step that accounts for the largest share of energy consumption in the entire primary aluminum production chain — making the choice of energy sources a major factor in the metal’s environmental footprint.
Alongside primary production exists the secondary route, which involves the remelting of aluminum and the recovery of aluminum through scrap recycling. Although aluminum itself can be recycled indefinitely without loss of quality, the primary source (bauxite) is finite and non-renewable in practical terms. Once the ore is mined and depleted, it cannot quickly be replaced by nature. That’s why recycling is so powerful: it keeps aluminum circulating in the economy and slows the depletion of these geological reserves, effectively extending their useful life.
Over the next decade, secondary aluminum supply is projected to grow at roughly twice the rate of primary production, reflecting the rising demand for recycled content.4 Importantly, post-consumer scrap carries a near-zero carbon footprint and requires only about 5% of the energy needed to produce primary aluminum. In response to customer expectations for more sustainable materials, the industry increasingly combines low-carbon primary aluminum — often produced with hydroelectric power — with recycled content. This approach offers a compelling solution that supports both environmental goals and market demand.
Trace Elements and Their Effect
A trace element is any element not intentionally added to an aluminum alloy. But how do these elements get there? Trace element pick-up is almost inevitable along the aluminum production chain — both in primary production and recycling.
Primary production introduces trace elements at several stages. Trace elements like Zn and gallium (Ga) come from raw materials, such as alumina, coke, and pitch, with their levels largely depending on the geological source of the bauxite. During electrolysis, additional elements such as Cu, Fe, and manganese (Mn) enter the melt through the bath chemistry, erosion of steel cathodes, and contact with steel equipment. Finally, in the casthouse, further contamination can occur from alloying additions, remelting of internal scrap, and the use of grain refiners.
Recycling also brings in trace elements from several sources. Post-consumer scrap typically carries Zn, Cu, and Fe, originating from coatings, lacquers, anodic layers, machining chips, and sometimes embedded contaminants, like screws or fasteners. While sorting technologies, spectroscopic analysis, and dilution with high-purity metal help control trace element levels, complete separation remains impossible — especially when dealing with large, mixed scrap streams.
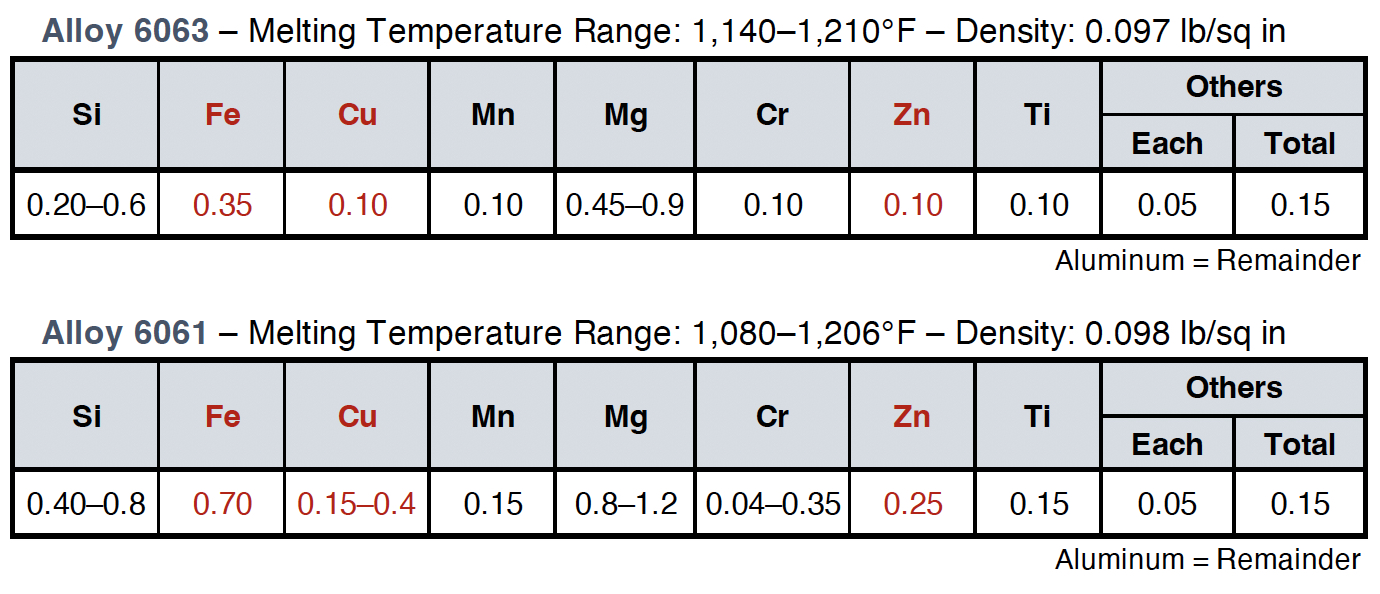
Even when the industry carefully specifies alloys, such as EN AW 6061 or 6063 (Table I), the real-world composition has to be carefully observed to make sure that a slightly higher content of trace elements is not introduced, if the feedstock is recycled. When it comes to corrosion and anodizing appearance, Zn, Cu, and Fe are the three most important trace elements to pay attention to (shown in red in Table I). Each of these elements can increase corrosion and surface defect risks in different ways. Zinc can form localized galvanic cells, accelerating pitting. Copper raises susceptibility to intergranular corrosion by forming Cu-rich particles. Meanwhile, iron forms intermetallic particles (e.g., AlFeSi) that become cathodic sites, leading to pitting under anodic films.
These trace elements can also alter the surface appearance after anodizing. For instance, iron particles may remain visible as dark streaks, while copper can lead to yellowish discoloration. The root cause of these effects lies in the microstructure of the surface being treated. Microstructure not only affects anodizing, because of the transparency of the oxide layer formed, but also influences paint adhesion — often manifesting as filiform corrosion. Furthermore, due to variations in the microstructure, there may be localized areas where the chemical conversion film or anodizing does not form adequately, increasing the risk of corrosion or visible defects.
Microstructure: The Hidden Factor
The way trace elements behave in the alloy depends heavily on the metallurgical history. For example, during the thermal cycles, heating the aluminum, to ~600°C during homogenization or extrusion alters diffusion of trace elements. Meanwhile, recrystallization changes grain size and orientation, increasing grain boundary area and diffusion paths.
In addition, the surface layers can show higher localized trace element concentrations even when bulk levels remain low. This localized enrichment matters most, as small amounts of Fe, Cu, or Zn at grain boundaries or near the surface can drastically reduce corrosion resistance, even if the alloy composition appears nominally within specification.
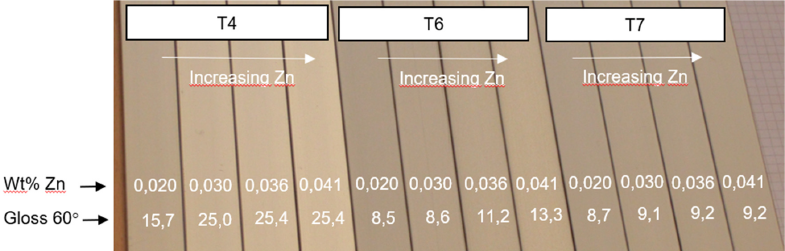
As a result, two billets of 6063 alloy with similar average Zn, Cu, and Fe can show very different corrosion and anodizing behavior, regardless of whether they are produced from primary or recycled aluminum. Bauger, et al., shows clearly that even a small amount of the trace element Zn (even much lower than the max. 0.1 and max. 0.25 wt% allowed value for 6063 and 6061, respectively) combined with variations in temperature can result in a huge difference in gloss and color (Figure 1).1
Post-Consumer Scrap
A growing trend among manufacturers and customers is to demand aluminum products made with 100% post-consumer scrap in order to meet ambitious sustainability targets. While the intention behind this is positive, it can also create confusion in the market—and, in some cases, drive costs unnecessarily high without delivering the environmental benefit people expect.
It is important to recall that aluminum’s true environmental strength lies in its durability and long service life. Most of the aluminum ever produced remains in use today,6 integrated into buildings, transportation systems, and other infrastructure that can last for decades. The flow of scrap back into recycling depends directly on how long products remain in service. Studies show that aluminum components in buildings have average lifespans of around 50 years, in automobiles about 15 years, and in beverage cans as little as a few months.7 This time lag fundamentally limits the immediate availability of post-consumer scrap, especially clean, high-quality scrap suitable for demanding applications.
As a result, there is simply not enough high-quality post-consumer scrap available to fully substitute primary aluminum, particularly for critical surface products like anodized extrusion and plate, where both chemical purity and visual consistency are essential, as mentioned before. Instead, much of today’s recycled content comes from process scrap or pre-consumer scrap—manufacturing waste produced during rolling, extrusion, or machining. While valuable, this type of scrap typically offers smaller carbon savings compared to true post-consumer scrap.8
Meanwhile, environmental product declarations (EPDs) and customer specifications increasingly demand high recycled content as a key measure of environmental performance.9 But if the volume of suitable post-consumer scrap is insufficient, the most effective first goal should often be reduction—designing products and systems that use less aluminum to begin with, have longer service lives, and are easier to recycle in the future. Such reduction strategies include smart design to lower part weight without compromising functionality; optimizing alloy selection for thinner sections, while maintaining required strength and corrosion performance; enhancing surface finishing processes to extend product life; developing new alloys and process controls that tolerate slightly higher trace element levels, thus enabling broader use of available scrap; and enabling higher and easier amounts of dismantling for products.
Focusing exclusively on recycled content risks overlooking this broader sustainability toolbox. In practice, the most impactful path combines recycled material—especially post-consumer scrap when available—with low-carbon primary aluminum produced using renewable energy, such as hydroelectric power. This balanced approach supports market expectations, helps meet tightening EPD and regulatory requirements, and most importantly, delivers real carbon reductions while preserving material quality and performance.
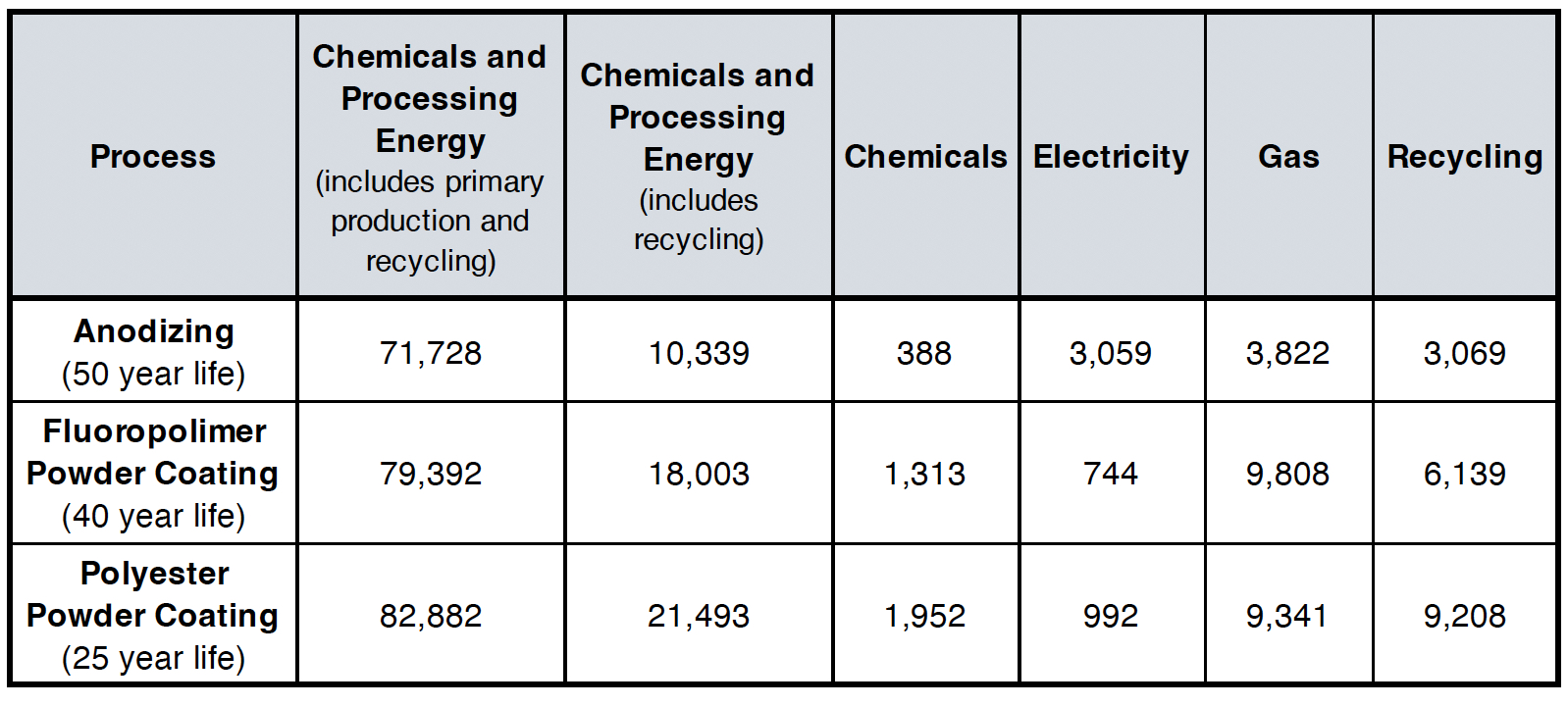
It is also worth adding a broader environmental perspective from the KMH report prepared for the Australian Anodizers Council.10 Their life cycle analysis compared anodizing with polyester and fluoropolymer powder coating, showing that anodizing generally has lower total energy consumption and greenhouse gas (GHG) emissions over a 100-year life cycle. This advantage comes largely from anodizing’s longer service life (estimated at ~50 years) and the fact that anodized aluminum is fully recyclable without losing quality or the need to introduce a “burn-off” stage before recycling as needed for lacquer to be removed. The study also highlighted that while powder coating processes may use slightly less direct electricity, they involve more energy-intensive chemicals and have a shorter service life, which increases total environmental impact when measured across multiple replacement cycles.
Table II illustrates the life cycle energy consumption per ton of aluminum production for anodizing and polyester powder coating processes. The analysis uses a 100-year life cycle, reflecting assumed service lives of 50 years for anodizing and 40 and 25 years for the powder coating processes. The total energy consumption is broken down into contributions from chemicals, electricity, gas, and the recycling process. It’s important to note that the substantial energy demand from primary aluminum production is included only when calculating the energy and GHG emissions associated with recycling, rather than counted multiple times across the product’s life cycle.
Despite all these advances, it is critical to remember that aluminum’s primary environmental advantage comes from its durability and recyclability over multiple life cycles. Products designed to remain in use longer often bring larger carbon savings than focusing solely on recycled content. For example, ensuring correct anodic film thickness and sealing quality directly improves corrosion resistance, color stability, and extends product lifespan — which in turn delays recycling and reduces total environmental impact.
Conclusion
Recycled aluminum can anodize just like primary aluminum—provided that alloy chemistry and process control are carefully managed. While the trace elements Zn, Cu, and Fe do influence corrosion behavior and surface appearance, factors such as production methods, heat treatment, and especially surface preparation often have an even greater impact on final performance.
Yet when we step back, the bigger picture becomes clear. First, there isn’t enough high-quality post-consumer scrap available today to meet growing demand, especially for products that require critical surface quality, such as architectural profiles and visible parts. Second, focusing solely on recycled content risks overlooking other impactful strategies: reducing material use through smarter design, extending product life through better finishing, and developing alloys that tolerate more recycled content without sacrificing performance. Third, EPDs should reflect total life cycle impact—not just the percentage of recycled content—rewarding longer-lasting solutions and low-carbon primary production.
In this broader context, anodizing stands out as an especially sustainable surface treatment. Unlike lacquers and powder coatings, anodizing becomes an integral part of the aluminum itself rather than just sitting on top as an organic film. This means it cannot peel or flake in the same way, maintains its metallic appearance over decades, and typically lasts longer before requiring renewal, as well as not requiring another process step, such as decoating, during recycling. As supported by life cycle studies such as those from the Australian Anodizers Council, anodized aluminum often shows a lower total energy demand and GHG footprint over its full life, thanks largely to its durability and the fact it can be fully recycled without losing its inherent qualities.
In short, the real challenge isn’t just controlling Zn, Cu, and Fe, but rather balancing quality, availability, and environmental goals. By designing smarter, finishing better, and thinking beyond recycled content alone, the aluminum industry can deliver products that truly last—and do so with a smaller environmental footprint.
References
- Bauger, Øystein, et al., “Influence of Trace Elements like Zn, Cu and Fe and Temper Conditions on Spangling and Gloss on Anodized Aluminum Profiles,” Presented at ET´ 22, Orlando, Florida, 2022.
- Ambat, Rajan, “Recycled aluminium alloys for sustainable performance: Some issues on corrosion and surface engineering,” ESTAL, 2023.
- Juhl, A.D., “Anodizing Recycled Aluminum: Revisiting 2009’s Challenges and Why They Persist Today,” Light Metal Age, June 2025.
- Tshipama, A., V. Goutière, and Pomerleau, “Opportunities for Adding Recycled Content to Primary Aluminum Products,” Engineering Proceedings, Vol. 43, No. 1, 2023, https://doi.org/10.3390/engproc2023043032.
- “North America Alloy Data Sheets,” Hydro.
- “Aluminum: The Element of Sustainability,” The Aluminum Association, September 2011.
- “Global Aluminium Recycling Flows,” International Aluminium Institute, 2021.
- Juhl, A.D., “Why Anodizing is the Most Sustainable Surface Treatment of Aluminum,” Light Metal Age, April 2022.
- “General Programme Instructions for Environmental Product Declarations: Version 4.0,” EPD International, 2021.
- “Environmental Impact of Fluoropolymer Powder Coating, Polyester Powder Coating and Anodising Processes,” KMH and Australian Anodising Association, November 2010.
Editor’s Note: This article first appeared in the August 2025 issue of Light Metal Age. To receive the current issue, please subscribe.