By Dr. George N. Oh, Nathan Sheffield, and Yechan Kang, Quaker Houghton, Metal Finishing Group.
Abstract
Quality of anodized aluminum, both mechanical and aesthetic, is affected by a number of parameters. Two parameters that have larger effects on finish and quality are anodize temperature and current density. This study shows that these parameters affect coating weight density, mechanical robustness (Michael-Clarke abrasion), electrolytic color tone, and even corrosion resistance. Using a well-established understanding of microstructural development of the anodic aluminum oxide film, this paper explains how and why these variations affect the resulting quality of the part. Control of just these two parameters goes a long way towards ensuring consistent results to meet specifications and customer requirements.
Introduction
Anodization of aluminum provides mechanical and chemical protection to aluminum metal and alloys by building a porous aluminum oxide layer, which is then sealed by chemical and/or physical methods.1-2 This layer is integral to the metal substrate and provides superior mechanical and chemical protection compared to paint, powder coating, or other coatings. Anodizing is fairly environmentally friendly, as it does not rely on hexavalent chromium, and it does not rely heavily on organic materials that must be waste-treated, as is typical for paint. One disadvantage of anodization compared to paint is that control of the final color of the finish is much more difficult. The depth of color is dependent on the amount of colorant taken up by the pores: adsorbed chromophore in the case of dyes, electrodeposited tin in the case of electrolytic color. Furthermore, interference phenomena can modulate the limited color tones of electrolytic color. As a result, moderate variations in the pore structure lead to small changes in color, which can nevertheless be detected by people with average to good color discrimination.
Like other finishing techniques, anodization requires understanding of a variety of factors that can cause problems with the final finish. Some, such as the role of effective cleaning, are common to all finishing techniques. Conversely, understanding of the underlying aluminum substrate is much more important for anodization than for other finishing techniques. And like all finishing techniques, some are particular to the technique. In this case, these include applied current in the anodize tank and temperature of the seal tank. Anodization particularly requires some breadth of knowledge, as it has more steps and process tanks than any other finishing process. Expert anodizers can control all of the operational parameters that affect anodize quality and appearance while being able to troubleshoot various problems. Maintaining control of basic anodizing factors goes a long way towards ensuring smooth operation and meeting standard specifications.
In this article, two parameters for the anodize tank will be focused on, namely current density and tank temperature. Their effects on a number of architectural specification requirements will be examined, connecting these effects to what is known about the microstructural development of the anodic aluminum oxide layer.
Experiment
Aluminum 6063-T6 panels were taken through a standard anodization process. The parts were cleaned, acid etched, caustic etched, deoxidized, anodized, and mid-temperature-sealed. Some parts were also electrolytically colored to a dark bronze. The finished panels were air-dried for at least 16 hours before analysis. Control panels were anodized at 18 ASF (A/ft2) and at 70°F for 28 minutes, targeting 0.7 mil thick coating. Additional panels were run at higher temperatures and at higher or lower current densities, adjusting the time according to the 720 Rule to target the same thickness.
Colors were measured in the L*a*b* color space using a Konica Minolta CM–700d Spectrophotometer in SCE mode.3 Color swatches were generated from the nix color sensor tool.4 ∆E values were measured using the Cie76 algorithm.5 The parts were subject to quality tests as per AAMA 611-14,6 with coating thicknesses measured using a Fischer MP0R Isoscope. The panels were also subject to acid dissolution tests (ADT) and Michael-Clarke abrasion tests. Coating weights and densities were also obtained. References contain the detailed experimental parameters and statistical treatment of data.7-9
Results
The results of the experiment determined the effects of temperature and current density on the specifications and color of the aluminum parts.
Effect on Specifications
It is generally recommended that a tank temperature of 70°F and a current density of 18 ASF be implemented during anodization. Some specification tests are fairly insensitive to variations in these parameters. Figure 1a shows the results of the ADT at 70, 80, and 85°F, and at 8, 13, 16, 18, and 24 ASF. The parts failed this test only at 8 ASF, and even then only at elevated temperatures. The ADT is designed to test the corrosion resistance of parts, which is primarily dependent on how well the pores generated during anodization are plugged, i.e. the efficacy of the sealing step. Conversely, the ADT depends relatively little on anodizing parameters, and only fails with gross deviations. The experiment showed that the optimum parameters for current density in this study are 70°F and 18 ASF.
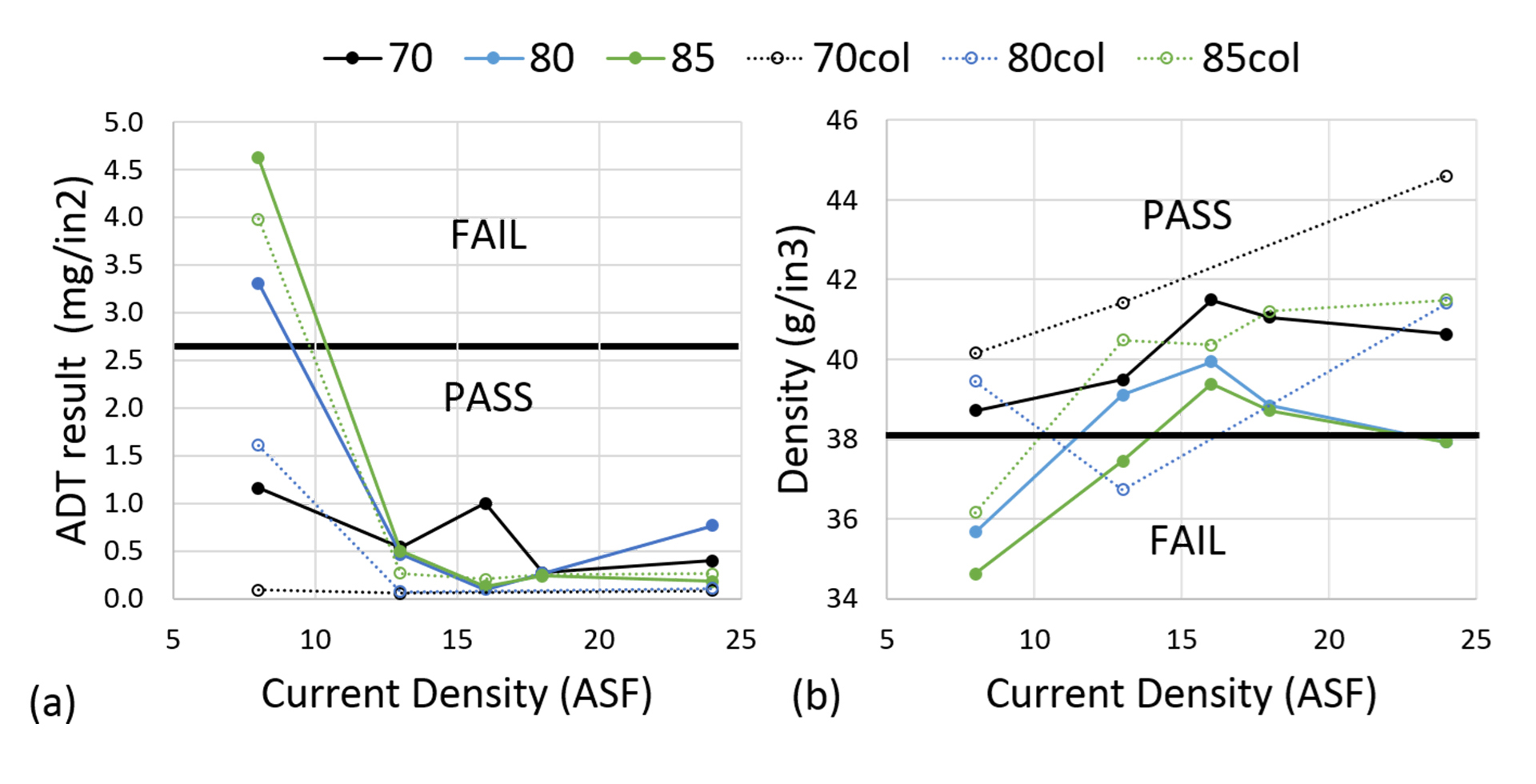
In contrast, coating weight density is significantly affected by variations, as it is highly dependent on the microstructure (Figure 1b). Let us examine the effects of varying current density during anodization: after the formation of the aluminum oxide barrier layer, close-packed hexagonal cells with a hollow center grow up from the substrate.2 The cell walls are composed of aluminum oxides. The central pore provides a constant source of oxide. Larger hexagonal cells arise from higher current densities, as the higher electronic polarization at the base of each cell repels other cells. This leads to fewer pores, more aluminum oxide, and an overall denser structure. However, the higher current density also leads to larger pore sizes, presumably to accommodate higher flow of electrolyte towards the surface, and by 24 ASF the pores are so large that the density of the coating goes down, even though the centers of the pores are further apart. While the current builds up the anodic aluminum oxide, the highly acidic bath slowly dissolves the aluminum oxide. This dissolution accelerates greatly at higher temperatures, and as a result, most parts run at 80 or 85°F fall below specification for coating weight density. Note that the coating weight density follows fairly consistent trends: at a given temperature, coating weight density goes up, and then down with greater current density for all temperatures. At a given current density, coating weight density goes down with temperature. For coating weight density, the optimum parameters are 70°F and 16 ASF.
The specification most sensitive to variations in tank parameters is the Michael-Clarke abrasion resistance test. Figure 2 shows that at 70°F, the parts anodized at 16, 18, and 24 ASF all pass. Meanwhile, at 80 and 85°F for all current densities, the mechanical robustness of the coating is poor. To justify these results, the microstructural arguments used to understand the coating weight density can be extended to the macroscopic scale by an analogy. Imagine a vast array of drinking glasses—sturdy, thick-walled tumblers could probably tolerate being walked on, but a collection of wine glasses would break immediately if stepped on. Lower current densities and higher temperatures result in the coating being built more slowly and dissolved more quickly, contributing to thinner pore walls, which fail the abrasion test. The optimum parameters for abrasion resistance are 70°F and 24 ASF.
Effect on Color
Temperature and current density variations also affect electrolytic color. These differences are evident not only by visual inspection (Figure 3), but also by looking at the L*a*b* values and the ∆E values between them (Table I). In general, parts anodized at higher temperatures or lower current densities have the lowest L* values and are the darkest in color. As a reminder, these conditions lead to larger pore diameter. In the electrolytic color tank, the pores are filled with sulfuric acid “wires.” Thicker wires have lower resistivity, and the result is faster electrodeposition and darker color.
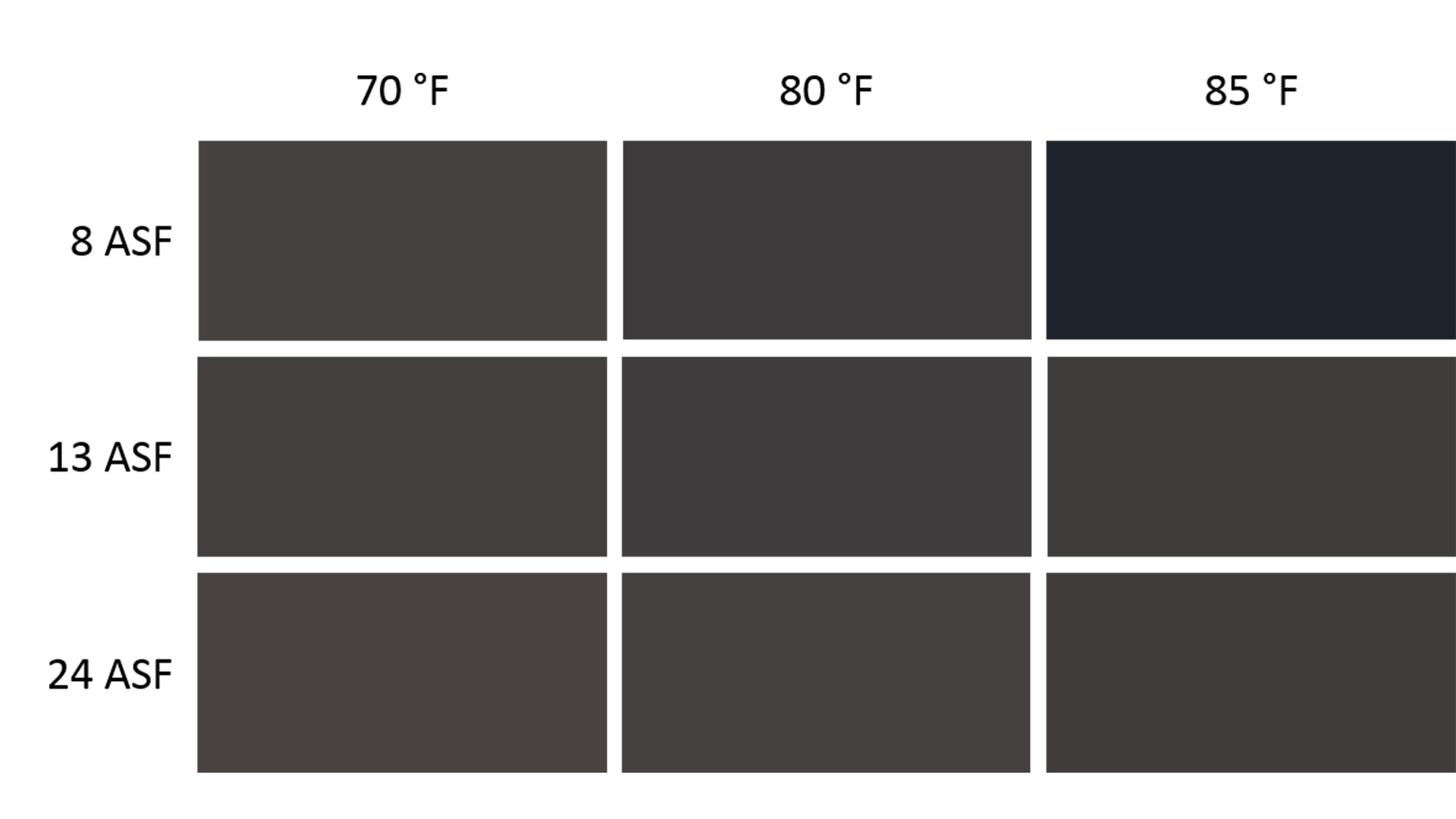
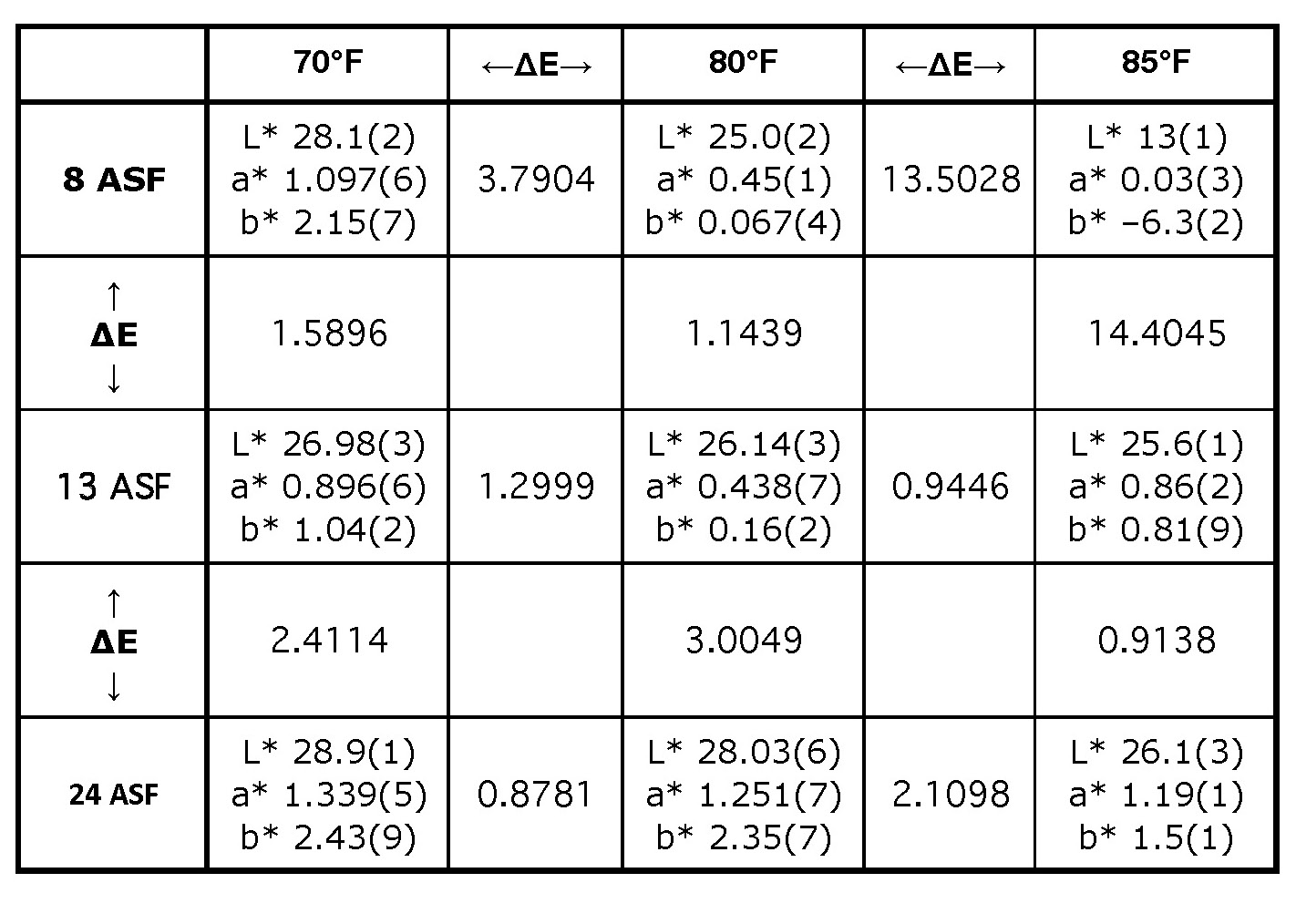
∆E is at best too reductive as a measure of color difference. In Figure 3, it is difficult to tell whether the center panel is closer in color to the center-top panel or the center-left panel. Their ∆E values are very similar, but because one varies on L* and the other on a*b*, the perception of the colors is quite different. Furthermore, people with less color discrimination ability might not be able to distinguish between these colors. Despite these issues, except when the microstructure is poorest, L*a*b* values do not vary independently, and when the level of darkness is well matched, the hue or shade will match reasonably well. Moreover, if the tank parameters are well controlled, the L*a*b* values are remarkably consistent, as seen by the standard errors of the measurements. At 70°F and 13 ASF, ∆E equals 0.03, which approaches the error of the measurement. This consistency of color does not include color effects from smut left on after caustic etch, a thick anodized aluminum layer, or the distribution of alloying elements, which differs between alloys and can vary even from the beginning to the end of a billet. These effects cause greater variations in color than moderate changes in the current density or temperature, and are the reason why the recommended color range is set to be noticeably broad, with AAMA-611 requiring ∆E < 5 for a sample set.
Conclusion
Anodizing at 70°F and 18 ASF ensures the largest amount of wiggle room to pass all of the specification tests. Without control of temperature and current density in the anodize tank, problems occur in a number of quality specifications. Abrasion resistance is the most sensitive, as would be expected due to the underlying defective microstructure. Color differences are also quite noticeable, though the numerous causes of color variations do not make color necessarily a useful diagnostic tool of problems in the process. To determine an acceptable color range, use of two color chips is still more useful than setting a ∆E in production. This is because, though a* and b* generally depend on L*, if ∆E is used as a benchmark, in the event of an unexpected large difference in a* and b*, it will be much more noticeable to the human eye than an identical ∆E with a larger L* difference.
References
- Brace, Arthur W. and Peter G. Sheasby, The Technology of Anodizing Aluminum, 2nd ed., Technicopy Ltd., Gloucestershire, England, 1979.
- Runge, Jude Mary, The Metallurgy of Anodizing Aluminum: Connecting Science to Practice, Springer International Publishing AG, Cham, Switzerland, 2018.
- “Specular Component Included (SCI) versus Specular Component Excluded (SCE),” Konica Minolta Sensing Americas, sensing.konicaminolta.us/blog/specular-component-included-sci-vs-specular-component-excluded-sce, data retrieved August 2018.
- “Color Converter Tool,” Nix Color Sensor, www.nixsensor.com/free-color-converter, data retrieved August 2018.
- Delta-E Calculator, colormine.org/delta-e-calculator, data retrieved June 2020.
- “AAMA 611-14: Voluntary Specification for Anodized Architectural Aluminum,” American Architectural Manufacturers Association (AAMA), Schaumburg, IL, 2014.
- Sheffield, Nathan, and George N. Oh, “Use of Spectrophotometers in Anodizing: Characterization of L*a*b* Readings and in Electro-Color Processes,” Aluminum Anodizers Council Meeting, Minneapolis, MN, 2018.
- Oh, George N. and Nathan Sheffield, “How Anodizing Quality Affects Follow-On Processes, Especially Sealing,” Aluminum Anodizers Council Meeting, Minneapolis, MN, 2018.
- Oh, George N. and Nathan Sheffield, “Anodizing by Current Density vs. Voltage,” Aluminum Anodizers Council Meeting, Houston, TX, 2019.
Editor’s Note: This article first appeared in the August 2020 issue of Light Metal Age. To receive the current issue, please subscribe.
George Oh is a R&D chemist at Quaker Houghton in the Metal Finishing Group, which he joined in 2017. He was previously a postdoctoral associate at the University of Houston and the University of Notre Dame, as well as a visiting scientist at the Max Planck Institute for Chemical Physics of Solids in Dresden, Germany. He obtained his B.A. in Chemistry and Japanese from Dartmouth College and a Ph.D. in Chemistry from Northwestern University. He currently serves as co-chair on the Aluminum Anodizers Council’s Education Committee.
Nathan Sheffield is a technical service chemist for Quaker Houghton in the Metal Finishing Group, which he joined in 2013. In this role, he functions as a technical consultant and educator for Quaker Houghton’s metal finishing business. He obtained his B.S in Chemistry at Kennesaw State University. He is a member of the Aluminum Anodizers Council and is currently serving on the Education Committee where he conducts educational classes on behalf of the council. He has received the “Robert L. Kersman Award of Excellence for Best Paper” in 2018.
Yechan Kang has been a R&D chemist at Quaker Houghton in the Metal Finishing Group since 2019. In this role, he conducts research in the anodizing process, focusing on consumption, color, and formulation. He obtained his B.S. in Biology and Chemistry within three years from Georgia Gwinnett College.