By S. Tichy, K. Büchner, S. Wibner, and H. Antrekowitsch, Montanuniversität Leoben, Chair of Nonferrous Metallurgy; and P. Pucher and B. Prillhofer, AMAG casting GmbH.
Natural gas is commonly used as an energy source in aluminum recycling furnaces, particularly for achieving high melting throughputs. While the direct use of electrical energy has the potential to support decarbonization, limitations such as challenges in handling organics persist. An alternative approach is to replace natural gas as fuel with hydrogen (H2).
AMAG Austria Metall AG successfully conducted large-scale industrial trials utilizing hydrogen as an energy carrier in its two casthouses. Firstly, hydrogen was employed in a rotary tilting furnace to melt low-grade scrap with the addition of salt. Secondly, clean scrap was melted with hydrogen in a single-chamber furnace to produce a 5xxx wrought alloy. To ensure statistical significance, reproducibility, and reliability, all tests were repeated multiple times. Reference batches using natural gas under otherwise identical process conditions facilitated an isolated evaluation of hydrogen’s influence on melt purity and quality, as well as on residues and off-gas emissions.
The planning and execution of the trials were grounded in several years of research conducted at laboratory and pilot scale, focusing on the suitability of various technologies for achieving climate neutrality, with particular emphasis on the use of hydrogen as a fuel.
The results are promising, demonstrating that hydrogen combustion is a viable option for the future decarbonization of aluminum recycling plants. However, it is important to note that the use of hydrogen introduces certain changes to the melting process compared to natural gas. Through its research-driven approach, AMAG can sustain its high product quality while achieving significant reductions in CO2 emissions. To gain deeper insights, a second hydrogen campaign is scheduled in the future, with a particular focus on oxide formation during the melting step.
Company Background
As part of the AMAG Group, AMAG casting GmbH in Ranshofen, Austria, is a leading producer of sustainable casting and wrought aluminum alloys in Europe, serving a wide range of industries. The company is deeply committed to environmental protection and the sustainable use of resources. Over the past several years, AMAG has focused on reducing energy consumption and CO2 emissions across its entire value chain. Specific CO2 emissions have already been lowered through initiatives, such as utilizing electricity from renewable sources. To cement its position as a pioneer in greenhouse gas reduction, AMAG casting GmbH aims to transform its entire plant park to CO2-neutral technologies by 2040/50. Therefore, the company is already taking all the necessary steps and measures to be ready to implement the changes in a targeted and scientifically sound manner.
Why Hydrogen?
Currently, the majority of industrial furnaces used for melting aluminum scrap rely on the combustion of natural gas (with its main component being methane, CH4). While the combustion of 1 Nm3 of CH4 (35.3 ft3) generates approximately 10 kWh (34,000 BTU) of heating capacity, it also produces 1 Nm3 of CO2 (35.3 ft3). Consequently, various melting and holding processes emit approximately 0.3 t CO2e/t Al (0.3 lb CO2e/lb Al).1 To reduce these emissions, alternative heating concepts are required. The most efficient option, both ecologically and economically, is the direct application of electrical energy. Induction furnaces present a viable solution for melting clean, dry, and fine aluminum scrap, with capacities reaching up to 70 tonnes (150,000 lbs). However, these furnaces are unsuitable for processing scrap containing organic contaminants. In the long term, plasma burners could emerge as a promising alternative. In the short-to-medium term, however, their adoption is hindered by high investment costs, estimated at approximately $500,000 to $1 million/MW of burner capacity, excluding the additional expenses associated with the necessary electrical plant infrastructure.2
A straightforward and cost-effective approach to decarbonize most furnaces involves adapting gas control systems and burners for hydrogen use. During a transitional phase, blending hydrogen with natural gas can facilitate a gradual reduction in greenhouse gas emissions. From a process management standpoint, substituting natural gas with hydrogen necessitates manageable adjustments. By precisely controlling the combustion stoichiometry, the oxygen partial pressure within the furnace can be kept low, and organic residues can be utilized for additional energy input. However, a change in the composition of the furnace atmosphere is unavoidable. This results from off-gases generated by the combustion of the respective fuel. The use of hydrogen eliminates CO2 emissions but increases water vapor concentration in the furnace atmosphere. For instance, in air burners, the water vapor content rises from 19% with natural gas to 35% with hydrogen, while in oxyfuel burners, the water vapor content increases from 67% with natural gas to 100% with hydrogen.3
Planning and Preparation of Trials
Since 2021, AMAG has been financing and coordinating two PhD research projects at the Chair of Nonferrous Metallurgy at Montanuniversität Leoben, Austria. These projects focus on the product- and process-specific effects of converting industrial furnaces from natural gas to hydrogen. The increased partial pressure from water vapor in the furnace atmosphere, resulting from hydrogen combustion, is expected to induce various changes, theoretically impacting several aspects, including hydrogen absorption of the melt, dross formation in melting and holding processes, pyrolysis behavior when melting scrap with organic residues, the dew point limit in the refractory material, and emissions.
The research has already provided valuable insights into the behavior and effects of hydrogen combustion within the process. Selected findings from laboratory- and pilot-scale investigations have been published and are therefore not elaborated upon in this context.4–9
Given the complexity of processes in industrial furnaces, not all factors can be fully taken into account in laboratory or pilot-scale tests. Therefore, a comprehensive evaluation of the impact of an alternative heating concept can only be achieved through trials conducted directly in production facilities. Such investigations also enable the validation of findings from basic research under industrial operating conditions.
Based on the preliminary work conducted at a small scale and addressing open research questions, two industrial test campaigns were planned and executed at AMAG casting in Ranshofen. The melting of low-grade scrap with the addition of a salt mixture was conducted in a rotary tilting furnace with an oxygen burner specifically adapted for hydrogen operation (Figure 1). In a single-chamber furnace, clean scrap was processed with an hydrogen-ready cold-air burner setup (opening photo). Since virtually all process steps of aluminum recycling can be carried out in these two furnaces, they are well suited for evaluating the use of hydrogen. However, it is worth noting that the single-chamber furnace, designed primarily as a casting furnace, exhibits lower melting rates compared to a conventional melting furnace.

Trial Procedure and Hydrogen Supply
Unlike natural gas, a large-scale supply infrastructure for hydrogen is not yet available. For the trials, the alternative fuel was delivered via truck using hydrogen trailers (Figure 2). With a gas demand of approximately 650 Nm3 H2/h (23,000 ft3 H2/h) and a trailer capacity of 4,000 Nm3 (141,000 ft3), trailer changes were necessary every six hours. To ensure continuous operation, two trailers were utilized, with one serving as a backup. In the event of a gas shortage in the primary trailer, the system allowed seamless switching to the secondary trailer. The hydrogen was transported from the trailers to the furnaces through approved hoses, a dedicated pressure-reducing station, and an adapted control system. Burners specially optimized for hydrogen ensured efficient combustion of the fuel. The system also retained the flexibility to switch back to standard natural gas operation for conduction of the reference batches at any time.

To ensure statistically significant results, five trials per test series were conducted using hydrogen, with an equal number performed using natural gas under otherwise identical process parameters. The selection of scrap prioritized high reproducibility and comparability within the test series. In the single-chamber furnace, a mixture of large and small piece 5xxx material was used, while in the rotary tilting furnace, one test series was conducted with high-oxidic material (dross) and another with organic-containing mixed scrap, both with the addition of salt. The primary objectives included monitoring melt purity and quality, as well as assessing residual materials and emissions. Additionally, economic factors such as energy demand and metal yield were also evaluated.
Results
When analyzing gas or energy demand, the decreased volume-related calorific value of hydrogen, which is approximately 3.3 times lower than that of methane (CH4), must be taken into account. Converting the energy consumption to a CH4-equivalent reveals that the actual energy demand for heating with hydrogen is comparable to that with natural gas, irrespective of the furnace type. However, the fuel costs are significantly higher when hydrogen is used.
A metallurgically and economically critical aspect of the investigations is the metal yield and the oxidation losses of metallic aluminum associated with the use of hydrogen as an alternative fuel. While the effects of oxygen (O2) and carbon dioxide (CO2) in the furnace atmosphere have been extensively studied, the influence of increased water vapor content resulting from hydrogen combustion is still largely unexplored. Oxygen in the furnace atmosphere promotes oxidation, whereas CO2 exerts an oxidation-inhibiting effect. In the rotary tilting furnace, metal yield remains unaffected by elevated water vapor contents. In contrast, the single-chamber furnace exhibits significantly higher dross formation during scrap melting in a hydrogen combustion atmosphere (Figure 3). However, in subsequent process steps, such as heating, alloying, and holding, no discernible influence of the energy source is observed. It is important to note that the melting intervals in the single-chamber furnace, which is typically used as a casting furnace, are significantly longer than those in conventional melting furnaces due to its reduced heating capacity. Potential factors contributing to the increased dross formation include changes in the coagulation behavior of aluminum droplets during melting, the absence of the oxidation-inhibiting effect of CO2, and the formation of unfavorable flow conditions or hotspots during hydrogen burner operation. This will be further investigated in the second set of large-scale industrial trials.

To quantify the impact of hydrogen combustion on the hydrogen absorption of the aluminum melt, hydrogen content measurements were performed in the liquid metal during all stages of wrought alloy production. The progression of this quality-critical parameter across the individual process steps is illustrated in Figure 4. Within the furnace, hydrogen absorption depends on both the melt temperature and the water vapor partial pressure in the furnace atmosphere. Shortly after melting the feedstock, the hydrogen content in the liquid aluminum remains low, irrespective of the fuel used.
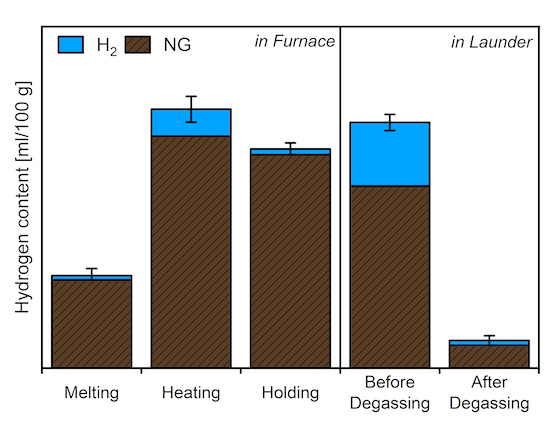
During heating and alloying, however, significant hydrogen absorption occurs, with hydrogen combustion resulting in approximately 10% higher levels compared to standard natural gas operation. A subsequent holding phase of several hours reduces the hydrogen content in the melt. During this phase, reduced heating capacity is required, leading to frequent burner operation in ignition or partial load mode. This lowers the partial pressure from water vapor and shifts the theoretical equilibrium concentration. When the melt is heated to casting temperature, hydrogen absorption increases again, particularly during hydrogen combustion. The average hydrogen content in the launder before degassing is approximately 35% higher for hydrogen batches compared to natural gas batches.
During the degassing treatments, the fuel-dependent difference decreases. Higher initial hydrogen levels improve degassing efficiency in the beginning for thermodynamic reasons. Nonetheless, after degassing, the hydrogen content in melts from hydrogen combustion remains slightly higher than in those from natural gas operation. These findings highlight the need for sufficiently dimensioned degassing systems to maintain product quality in the event of a transition to hydrogen combustion. For melting scrap containing organics or high oxide content in the rotary tilting furnace, the use of hydrogen as a fuel also tends to increase hydrogen absorption. However, in this case, the salt slag acts as a barrier, mitigating the effect.
The second critical parameter influencing melt quality is the presence of non-metallic inclusions. Analytical results confirm that the use of hydrogen as a fuel does not alter the type or quantity of these inclusions.
Depending on furnace type and process, the residual material is either salt slag or dross, with the latter being recycled directly at AMAG. Salt slag requires external processing, as the material can react with water and must therefore not be landfilled. Detailed chemical analyses indicate that the altered atmosphere during hydrogen combustion has no adverse effect on the composition or gas formation potential of the residual materials.
Analyses of process off-gases reveal only minor changes associated with hydrogen combustion. As expected, the transition leads to the desired elimination of burner-based CO2 emissions. Total organic carbon (TOC) and sulfur dioxide (SO2) levels remain within the permissible range, similar to those observed with natural gas burners. Emissions of hydrogen fluoride (HF), hydrochloride (HCL), and hydrocyanic acid (HCN) are near the detection limits. Additionally, critical compounds—such as dioxins and furans (PCDD/F) and benzo(a)pyrene (B[a]P)—are well below regulatory thresholds. However, a notable drawback of hydrogen combustion is an increase in nitrogen oxide (NOx) concentrations in the off-gas. This results from the elevated formation of thermal NOx, driven by higher flame temperatures during hydrogen combustion.
Conclusion
The results demonstrate that the use of hydrogen instead of natural gas as an energy source is an effective and technically feasible pathway for decarbonization. This approach enables a significant reduction in CO2 emissions, while maintaining high process and product quality. The energy efficiency of the furnaces remains unchanged when switching from natural gas to hydrogen, although this does not account for the significantly higher fuel costs of hydrogen. However, the partially observed changes in oxidation behavior during melting present a challenge. A reduction in metal yield could substantially impact the economic viability of the process. Therefore, this issue requires further detailed investigation, ideally in a dedicated melting furnace equipped with appropriate heating capacity.
Nonetheless, there is still a long way to go before this potentially climate-neutral energy source can be feasibly integrated into standard production. A reliable hydrogen supply for industrial applications necessitates the development of appropriate infrastructure, ideally a dedicated pipeline network. Continuous delivery via truck trailers is not a viable solution, as a single hydrogen trailer provides sufficient gas for melting only around 10 tonnes (22,000 lbs) of aluminum scrap. The reduction of NOx emissions during hydrogen combustion primarily falls within the scope of burner system manufacturers. Encouragingly, several promising approaches have already been developed to address this issue.
References
- “A low carbon footprint,” European Aluminium, accessed on October 14, 2024.
- Prillhofer, B., “Technology assessment of the transition to a carbon neutral foundry,” Presented at NorCast – 17th Nordic Aluminium Casthouse Conference, Arendal, Norwegen, 2024.
- Fragner, W., B. Prillhofer, and P. Pucher, “Die Transformation zur klimaneutralen Gießerei – Technologieevaluierung,” International Aluminium Journal, Vol. 12, 2023, pp. 36–40.
- Büchner, K., “Investigation of the influence of furnace atmospheres on the pyrolysis behaviour of coated aluminum scrap,” Presented at the GDMB Light Metals Expert Committee, Maasmechelen, Belgien 2024.
- Büchner, K., et al., “Investigation of the influence of different parameters on the pyrolysis of coated aluminium scrap by thermogravimetry and optical evaluation,” Proceedings of the European Metallurgical Conference (EMC 2023), GDMB Gesellschaft der Metallurgen und Bergleute e.V, 2023, Clausthal-Zellerfeld, Germany, pp. 321–335.
- Tichy, S., et al., “Influence of Water Vapor on the Oxidation Behavior of Molten Aluminum Magnesium Alloys,” Light Metals 2024, Springer, pp. 890–896.
- Tichy, S., “Mechanism of hydrogen absorption from H2O in aluminium melts,” Presented at the GDMB Light Metals Expert Committee, Maasmechelen, Belgium, 2024.
- Tichy, S., et al., “Hydrogen Absorption of Aluminum-Magnesium Melts from Humid Atmospheres,” Light Metals 2023, Springer Nature Switzerland, pp. 920–927.
- Tichy, S., et al., Comparison of experimental methods to investigate the oxidation behaviour of aluminium melts,” Proceedings of the European Metallurgical Conference (EMC 2023), GDMB Gesellschaft der Metallurgen und Bergleute e.V, 2023, Clausthal-Zellerfeld, Germany, pp. 833–844.
Editor’s Note: This article first appeared in the June 2025 issue of Light Metal Age. To receive the current issue, please subscribe.